Imaging Pearls ❯ Kidney ❯ Retriperitoneal Masses
|
-- OR -- |
|
- IMPORTANCE Testicular cancer is the most common solid malignancy among males aged 15 to40 years in the US, with approximately 10 000 new cases diagnosed each year. Between 90% and 95%of testicular cancers are germ cell tumors (GCTs).
CONCLUSIONS AND RELEVANCE Testicular cancer is the most common solid malignancy in young men in the US, and 90% to 95%are GCTs. Patients with testicular GCT have a 5- year survival rate of 99%, 92%, and 85%for stages I, II, and III, respectively. Prompt diagnosis and treatment are important to optimize outcomes, and treatment decisions should balance oncologic control with survivorship concerns to minimize long-term adverse effects of treatment.
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - The mean age at diagnosis for testicular cancer is 33 years. GCTs are categorized as seminomas and nonseminomatous GCTs (NSGCTs) based on their embryonic origins and path of differentiation. Risk factors include cryptorchidism, family history of testicular cancer, gonadal dysgenesis, infertility, cannabis use, and genetic conditions such as Klinefelter syndrome. The most common presenting symptom of testicular cancer is a painless testicular mass. History, physical examination, scrotal ultrasound, laboratory assessment of GCT-associated serum tumor markers (α-fetoprotein, β-human chorionic gonadotropin, and lactate dehydrogenase), and prompt referral to a urologist are indicated when testicular cancer is suspected.
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - “The most common presenting symptom of testicular cancer is a painless testicular mass. History, physical examination, scrotal ultrasound, laboratory assessment of GCT-associated serum tumor markers (α-fetoprotein, β-human chorionic gonadotropin, and lactate dehydrogenase), and prompt referral to a urologist are indicated when testicular cancer is suspected. Early diagnosis and treatment, starting with a radical inguinal orchiectomy, are important to optimize outcomes. At diagnosis, GCT is stage I (localized to the testicle) in 70%to 75%of patients, stage II (metastatic only to the retroperitoneal lymph nodes) in 20%, and stage III (widely metastatic) in 10%. Treatment of GCTs is guided by histology, clinical staging, and risk classification, with 5-year survival rates of 99%, 92%, and 85%for those diagnosed at stages I, II, and III, respectively. Optimal treatment often involves a multidisciplinary team at high-volume, experienced medical centers and may include surveillance (serum tumor markers [α-fetoprotein, β-human chorionic gonadotropin, and lactate dehydrogenase] and imaging of the chest, abdomen, and pelvis), surgery (retroperitoneal lymph node dissection), chemotherapy, and/or radiation.”
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - “In 90% of patients with testicular cancer, the presenting symptom is a testicular abnormality,34 most commonly a painless testicular mass identified incidentally or after minor testicular trauma that may prompt a patient to become aware of a testicular mass. Approximately 10% of patients with testicular cancer have acute testicular pain attributable to rapid tumor growth that may cause intratesticular hemorrhage, testicular infarction, or rarely testicular torsion. Scrotal swelling, heaviness, discomfort, and testicular shrinkage are less common (<10%) manifestations of testicular cancer.”
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - “In 10% to 20%of patients, initial symptoms of testicular cancer result from metastatic disease. Spread of testicular cancer to the retroperitoneal lymph nodes may present as a palpable abdominal mass, which can obstruct the ureters causing flank pain; compress or invade the bowel causing gastrointestinal symptoms such as nausea, bloating, or constipation; impede venous blood flow from the testis causing a varicocele; or invade the spinous muscles or nerves causing back pain. Pulmonary metastases, which occur in 5%of patients with testicular cancer,may present as dyspnea, cough, chest pain, or hemoptysis. Palpable neck masses in young men may be caused by testicular cancer metastasis to supraclavicular lymph nodes.”
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - “The life expectancy of men diagnosed with testicular cancer at 30 years of age is estimated at 75.2 years, which is 1.3 years less than men without cancer. Across all stages of testicular cancer, 5-year overall survival is 95% (99%for stage I, 92%for stage II, and 85% for stage III). Among patients with metastatic disease, 5-year progression-free and overall survival vary by IGCCCG prognostic group: for the good risk IGCCCG group, progression-free survival is 89% to 90% and overall survival is 96%; intermediate risk has 77% progression-free and 88% overall survival; and poor risk has 54% progression-free and 67% overall survival.”
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. - Testicular cancer is the most common solid malignancy in young men in the US, with a mean age of 33 years at diagnosis. Approximately 90% to 95% are GCTs. Management and prognosis of testicular cancer are determined by histology and clinical stage. Patients with testicular GCTs have a 5-year survival rate of 99%, 92%, and 85%for stages I, II, and III, respectively. Prompt diagnosis and treatment are paramount to optimizing outcomes, and treatment decisions should balance oncologic control with survivorship concerns to minimize long-term adverse effects of treatment.
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803. 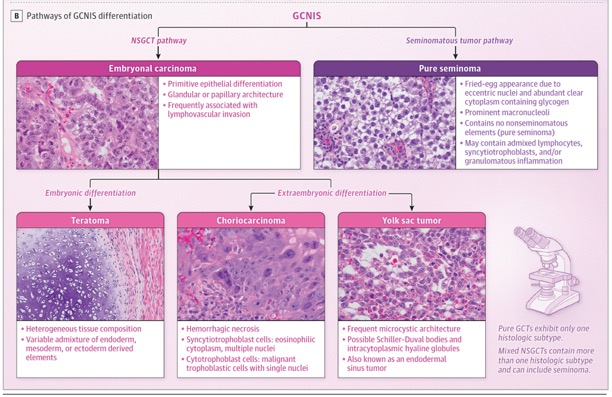
Testicular Germ Cell Tumors: A Review.
Singla N, Bagrodia A, Baraban E, Fankhauser CD, Ged YMA.
JAMA. 2025 Mar 4;333(9):793-803.
- “The perirenal space is a retroperitoneal space that is limited anteriorly by the anterior renal fascia (Gerota fascia) and posteriorly by the posterior renal fascia (Zuckerkandl fascia).These two fasciae fuse to form the lateroconal fascia laterally and blend loosely with the periureteric connective tissue medially. Superiorly, the two fasciae are fixed to the diaphragmatic fascia above the adrenal glands; inferiorly, they blend with the iliac fascia.The anterior and posterior renal fasciae enclose a gradually tapering conelike space produced by the embryologic ascent of the kidneys from the pelvis to the adult retroperitoneal position.”
Neoplastic and Non- neoplastic Proliferative Disorders of the Peri-renal Space: Cross- sectional Imaging Findings
Surabhi VR et al.
RadioGraphics 2008; 28:1005–1017 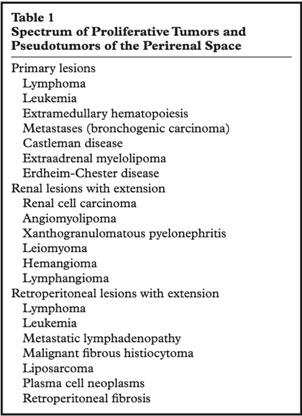
Neoplastic and Non- neoplastic Proliferative Disorders of the Peri-renal Space: Cross- sectional Imaging Findings
Surabhi VR et al.
RadioGraphics 2008; 28:1005–1017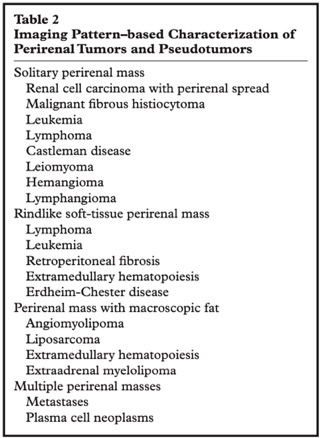
Neoplastic and Non- neoplastic Proliferative Disorders of the Peri-renal Space: Cross- sectional Imaging Findings
Surabhi VR et al.
RadioGraphics 2008; 28:1005–1017- “The perinephric space, shaped as an inverted cone, sits between the anterior and posterior renal fasciae. It can play host to a variety of clinical conditions encountered daily in the reporting schedule for a radiologist. Lesions may be classified and diagnosed based on their imaging characteristics, location and distribution. A broad range of differential diagnoses can be attributed to pathology sitting within this space, often without clinical signs or symptoms. An understanding of commonly encountered conditions affecting the perinephric space, along with characteristic imaging findings, can illustrate and often narrow the likely diagnosis.”
Radiological diagnosis of perinephric pathology: pictorial essay 2015
Goran Mitreski, Tom Sutherland
Insights Imaging (2017) 8:155–169
- Solid Retroperitoneal Tumors: Differential Dx
-Mesodermal neoplasms
-Neurogenic neoplasms
-Germ cell, sex cord, and stromal tumors
-Lymphoid and hematologic neoplasms - Mesodermal Neoplasms: Facts
-Liposarcoma, leiomyosarcoma and MFH make up more than 80% of these tumors
-Most common in 5th and 6th decades of life
-Often aggressive these tumors may be difficult to result and often recur locally or with metastases to liver, lung, bone, and brain - Liposarcoma of the Retroperitoneum: Facts
-Most common retroperitoneal sarcoma (33%)
-10-15% of all liposarcomas occur in the retroperitoneum
-Age range is usually 50-70 years of age
-Usually large with average size of 20 cm
-Four subtypes are well-differentiated, myxoid, pleomorphic and round cell. Well differentiated is most common in retroperitoneum - Retroperitoneal Leiomyosarcoma: facts
-Usually large (>10 cm) when discovered
-More common in woman and usually seen in the 5th to 6th decade of life
-On CT they are usually solid masses but may be cystic and necrotic as well
-6% of Leiomyosarcomas arise from the IVC and are primary in the IVC - Malignant Fibrous Histiocytoma (MFH) of the Retroperitoneum: Facts
-MFH is the most common sarcoma in the body and 15% arise in the retroperitoneum
-More common in male by 3-1 and most common in the 50-60 age group
-Mass may be solid or necrotic and may contain calcification in 7-20% of cases. This may be critical for the differential dx. - Other Retroperitoneal Sarcomas: Facts
-Rhabdomyosarcoma common in younger patients with bimodal peak at age 7 and then teen years
-Angiosarcoma, chondrosarcoma, synovial cell sarcoma all may occur but are rare. Synovial cell sarcoma usually occurs in patient 15-40 years of age which is younger than the other tumors

