Imaging Pearls ❯ Deep Learning ❯ Liquid Biopsy
|
-- OR -- |
|
- Objective: Multicancer Detection (MCD) tests, such as the GRAIL Galleri, offer a novel approach to cancer screening by detecting cancer-specific methylation patterns in cell-free DNA through a single blood sample. This study evaluated an 18-month implementation of MCD testing in a tertiary ambulatory internal medicine clinic.
Patients and Methods: Between June 2022 and November 2023, 2244 asymptomatic (without symptoms attributed to cancer) patients underwent MCD testing. The study focused on operational workflows, patient and physician education, and diagnostic follow-up of positive results. Standardized materials, including electronic health record (EHR) workflows, FAQs, and diagnostic pathways, were developed to facilitate implementation. Challenges included managing false positives, patient anxiety, costs, and ethical considerations.
Results: Of the 2244 patients tested, 17 (0.76%) had positive results, and 15 underwent further diagnostic evaluation. Cancer was confirmed in 11 (73.3%) patients, including cases of breast, colon, esophageal, lymphoma, ovarian, and pancreatic cancers. Four patients had no identifiable malignancy despite comprehensive work-up.
Conclusions: MCD testing is feasible in routine clinical workflows, with 73% of positive cases yielding cancer diagnoses. While promising, further research is required to assess long-term outcomes, cost-effectiveness, and optimal implementation strategies of cancer interception in broader healthcare settings.
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - “The concept of cancer interception is the proactive approach to identifying and intervening early in cancer development and progression to invasive disease. Currently, population-wide screening is only recommended for a few cancers, such as breast, colorectal, cervical, and (in high-risk individuals) lung. Cancers that have demonstrated increased mortality rates, such as pancreas and ovarian, have had no reliable biomarkers for interception or population-based screening tests in asymptomatic patients.”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - “Blood is inherently well suited as a potential biomarker source for cancer screening as it may contain circulating tumor cells, microRNA, and/or cell-free DNA (cfDNA) “shed” from a hitherto occult cancer. This potential has led to the development of novel blood-based, high-performance genomic technologies allowing for early detection of signals from multiple cancers, giving rise to a new paradigm of screening tests called multi-cancer detection (MCD) tests. MCD blood tests rely on analyses from cancer-specific DNA methylation patterns and have the potential to detect numerous cancer types in a single blood sample.”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 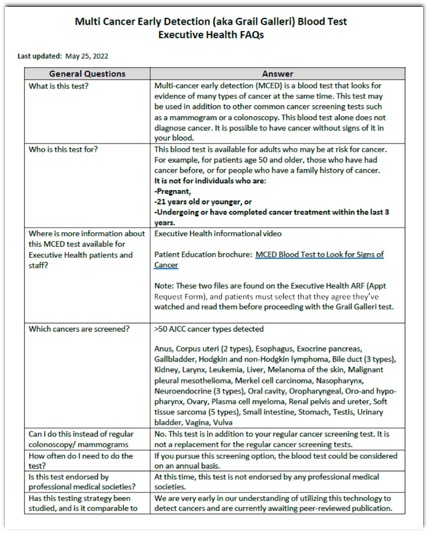
- The current MCD program has subsequently updated these recommendations using an iterative process involving institution-wide specialist opinion. In addition, a standardized Epic order set was developed to efficiently select the best initial test after a positive MCD result. In situations where the initial diagnostic evaluation did not yield an answer to the positive MCD signal, we strongly considered escalating to a full-body PET scan in many scenarios. For patients with a positive MCD and a negative workup (including negative PET) conducted at MC, we recommend a follow-up MCD test within 6 months (cost covered by the MCD company) with a subsequent workup if the signal persists.
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 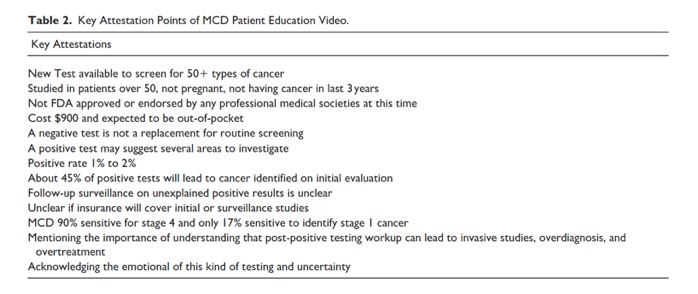
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18- “Of the 15 remaining MCD-positive patients who went on to receive complete diagnostic workups, 11 (73.3%) patients were found to have cancer, representing true positives. These cancers included breast (n = 1), colon (n = 2), esophageal (n = 1), lymphoma (n = 2), oropharyngeal, for example, glossotonsillar sulcus cancer (n = 1), ovarian (n = 1), pancreatic (n = 1), tonsillar (N = 1). Diagnostic evaluation for 4 patients with MCD-positive signals did not reveal a source of the signal. These patients received repeat MCD testing in 6 months, with the plan of repeating diagnostic workup if the testing remained positive. Of the 17 patients with positive MCD testing, 13 (76.5%) had more than 1 positive cancer signal localization (eg, head and neck, lung, lymphoid). In total, the 17 patients had 30 positive cancer signal localizations .”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - “The study demonstrated that less than 1% of patients in this population undergoing the test have a positive MCD test. Among those patients who tested positive, 73% were true positives with breast, esophageal, lymphoma, oropharyngeal, ovarian, and pancreas cancers being diagnosed. The remaining 27% of patients with a positive test and negative diagnostic work-up are being followed with a defined plan of repeat testing in 6 months and continued follow-up.”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - ”One of the challenges to the pragmatic implementation of MCD and other novel tests is the buy-in needed from primary care physicians (PCP). The Vanguard study was a pilot that utilized focus group evaluations to report on the perceptions of PCP and laypersons of different clinical trial designs and their willingness to participate. It was found that both primary care physicians and laypersons expressed concerns and reluctance about a study design in which MCD test results would not be returned to the Vanguard control group (intended effect).Still, they also noted that they were open to participation in MCD clinical trials if there was transparency regarding whether MCD testing would be run on provided biospecimens and if results would be eventually returned so that they could deliver those results to their patients. The findings of this study provided insights to guide clinical trial designs and plan prospective evaluation of MCD testing.”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - “The 18-month prospective cohort study highlights the feasibility of implementing MCD testing within a tertiary referral center. The findings demonstrate that MCD testing can effectively identify cancers in asymptomatic (without symptoms attributed to cancer) populations, with 73% of positive cases yielding a new cancer diagnosis. These results underscore the potential of MCD testing to complement existing cancer screening protocols, enabling earlier detection and intervention. However, challenges such as false positives, patient anxiety, cost considerations, and the integration of MCD testing into routine clinical practice require further investigation. As the role of MCD testing continues to evolve, its potential to transform cancer interception and improve patient outcomes remains a promising frontier in precision medicine.”
Implementation of a Multicancer Detection (MCD) Test in a Tertiary Referral Center in Asymptomatic Patients: An 18-Month Prospective Cohort Study
Ryan T. Hurt et al.
Journal of Primary Care & Community Health Volume 16: 1–18 - “Cancer is the leading cause of death globally, with nearly 20 million new cases and 9.7 million deaths in 2022. Due to its vague initial symptoms, cancer is often difficult to detect in its early stages. Liquid biopsy, a revolutionary approach in oncology, provides a minimally invasive, real-time method for cancer detection, monitoring, and characterization by examining circulating tumor components in body fluids. This review presents current technologies and clinical applications of liquid biopsy, focusing particularly on its value for early cancer diagnosis. Liquid biopsy enables molecular profiling of cancer for precision oncology by isolating circulating extracellular nucleic acids (cell-free DNA), circulating tumor DNA, and circulating tumor cells from blood and other body fluids. Cell-free DNA, which circulates freely in the blood, may or may not be tumor-derived, while circulating tumor DNA is specifically of tumor origin. Additionally, circulating tumor cells can be isolated from blood; these cells, shed from tumors into the bloodstream, typically survive only 1–2.5 h before immune clearance, though a small fraction can persist and metastasize to distant sites. Exosomes, small membrane-bound vesicles secreted by tumor cells, also carry molecular information about the tumor and have become a valuable source of biomarkers in liquid biopsy. Advances in detection technologies for these analytes have expanded the utility of liquid biopsy, facilitating the identification of somatic mutations and actionable genomic alterations in tumors.”
Liquid Biopsy: A Breakthrough Technology in Early Cancer Screening
Xuexin Liang, Qingqing Tang, Jiawei Chen and Yanghui Wei*
Cancer Screening and Prevention 2025 (in press) 
- “Blood-based tests present a promising strategy to enhance cancer screening through two distinct approaches. In the traditional paradigm of “one test for one cancer”, single-cancer early detection (SCED) tests a feature high true positive rate (TPR) for individual cancers, but high false-positive rate (FPR). Whereas multi-cancer early detection (MCED) tests simultaneously target multiple cancers with one low FPR, offering a new “one test for multiple cancers” approach. However, comparing these two approaches is inherently non-intuitive. We developed a framework for evaluating and comparing the efficiency and downstream costs of these two blood-based screening approaches at the general population level.”
Estimating the Burden of False Positives and Implementation Costs From Adding Multiple Single Cancer Tests or a Single Multi-Cancer Test to Standard-Of-Care Screening.
Madhavan S, Hackshaw A, Hubbell E, et al..
Cancer Med. 2025 Mar;14(6):e70776. doi: 10.1002/cam4.70776. - Methods: We developed two hypothetical screening systems to evaluate the performance efficiency of each blood-based screening approach. The “SCED-10” system featured 10 hypothetical SCED tests, each targeting one cancer type; the “MCED-10” system included a single hypothetical MCED test targeting the same 10 cancer types. We estimated the number of cancers detected, cumulative false positives, and associated costs of obligated testing for positive results for each system over 1 year when added to existing USPSTF-recommended cancer screening for 100,000 US adults aged 50–79.
Results: Compared with MCED-10, SCED-10 detected 1.4x~ more cancers (412 vs. 298), but had 188x~ more diagnostic investigations in cancer-free people (93,289 vs. 497), lower efficiency (positive predictive value: 0.44% vs. 38%; number needed to screen: 2062 vs. 334), 3.4x~ the cost ($329 M vs. $98 M), and 150x~ higher cumulative burden of false positives per annual round of screening (18 vs. 0.12).
Estimating the Burden of False Positives and Implementation Costs From Adding Multiple Single Cancer Tests or a Single Multi-Cancer Test to Standard-Of-Care Screening.
Madhavan S, Hackshaw A, Hubbell E, et al..
Cancer Med. 2025 Mar;14(6):e70776. doi: 10.1002/cam4.70776. - Conclusions: A screening system for average-risk individuals using multiple SCED tests has a higher rate of false positives and associated costs compared with a single MCED test. A set of SCED tests with the same sensitivity as standard-of- care screening detects only modestly more cancers than an MCED test limited to the same set of cancers.
Estimating the Burden of False Positives and Implementation Costs From Adding Multiple Single Cancer Tests or a Single Multi-Cancer Test to Standard-Of-Care Screening.
Madhavan S, Hackshaw A, Hubbell E, et al..
Cancer Med. 2025 Mar;14(6):e70776. doi: 10.1002/cam4.70776. 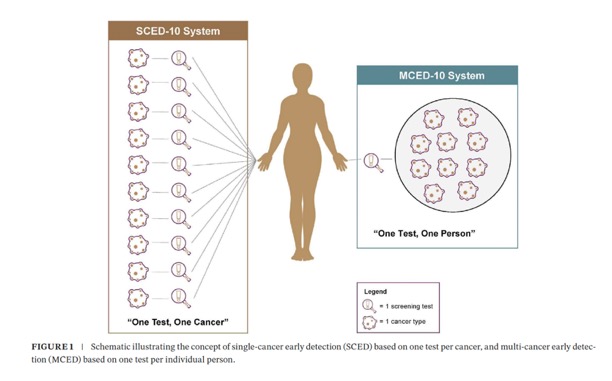
Estimating the Burden of False Positives and Implementation Costs From Adding Multiple Single Cancer Tests or a Single Multi-Cancer Test to Standard-Of-Care Screening.
Madhavan S, Hackshaw A, Hubbell E, et al..
Cancer Med. 2025 Mar;14(6):e70776. doi: 10.1002/cam4.70776.- As shown in our analysis, an MCED test that is limited to target the same 10 cancer types would detect 27% fewer incremental cancers than multiple SCED tests but is more efficient because this screening system would have substantially fewer incremental false positives—approximately 400 for MCED-10 versus nearly 100,000 for SCED-10.Because this comparison was based on the same 10 cancer types, it demonstrates clearly that a single MCED test simultaneously covering multiple cancer types is more efficient than implementing multiple SCED tests. This comparison illustrates that a single MCED test with a single FPR, as opposed to multiple FPRs from multiple independent SCED tests, is far more efficient and should be more cost-effective in a general population setting.
Estimating the Burden of False Positives and Implementation Costs From Adding Multiple Single Cancer Tests or a Single Multi-Cancer Test to Standard-Of-Care Screening.
Madhavan S, Hackshaw A, Hubbell E, et al..
Cancer Med. 2025 Mar;14(6):e70776. doi: 10.1002/cam4.70776. - Evaluation of genetic information leading to early cancer diagnosis and management involves analysis of cell-free DNA analyzed via liquid biopsy through various laboratory methods. Cell-free DNA (cfDNA) refers to DNA fragments found freely in body fluids that can be derived from both healthy and cancerous cells. Fragments of cfDNA derived from healthy cells average around 160 base pairs in length, and are distinguishable from tumor-associated cfDNA, termed circulating tumor DNA (ctDNA), which averages a slightly shorter fragment length of 140 base pairs
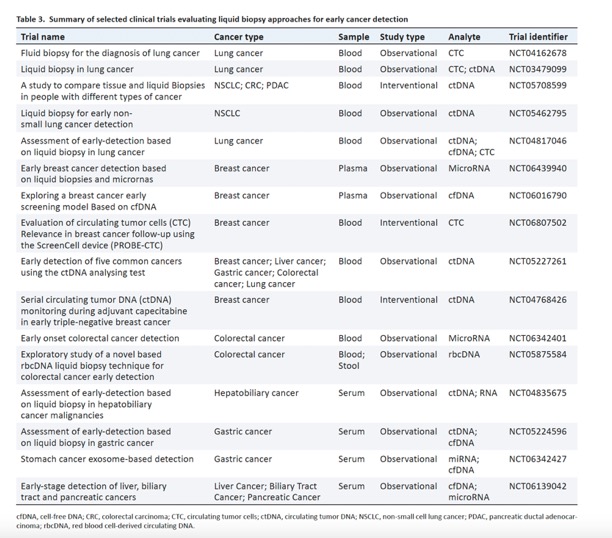
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - The consensus on the origin of ctDNA involves primary tumor cells that have undergone apoptosis, necrosis, or are actively metastasizing. In addition to ctDNA, circulating tumor cells (CTCs) as well as other cellular components such as RNA or proteins may be present and provide information indicative of malignancy and of the primary tumor’s characteristics . With a half-life of less than 2 hours, ctDNA analysis provides an accurate reflection of the current genetic landscape and behavior of the primary tumor in real-time. From first identification of its relevance in cancer patients in the landmark paper by Leon et al. in 1977, ctDNA has grown in its significance to be at current the most established liquid biopsy analyte for use in cancer management with multiple large clinical trials and FDA-approved device panels evaluating its validity in early diagnosis, prognostication, targeted treatment selection, and residual disease monitoring.
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - “Early diagnosis of PDAC is difficult for many reasons. For one, screening of individuals at average risk for pancreatic cancer is not standard of care. Around 70-90% of individuals who will be diagnosed with PDAC are at average risk . Many of the most common factors are only weakly associated with an increase in risk and are largely related to a patient’s lifestyle (e.g. smoking use, uncontrolled diabetes, alcohol use, obesity). Screening for PDAC often involves time-consuming and expensive procedures such as yearly MRI, esophagogastroduodenoscopy and endoscopic ultrasound, repeat fine needle aspirations and blood draws that have not been shown to identify enough early PDAC cases to justify the extent of invasive procedures that individuals will undergo throughout their lifetime .”
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - Monitoring of pancreatic intraepithelial neoplasms (PanINs), histologically identified areas within the pancreas that in rare cases progress to malignancy, is also not a possibility as these lesions are not directly evaluable except in pathologic specimens, and data is lacking to clearly understand the percentage of patients with PanINs that will progress to PDAC. Some studies have estimated the likelihood of PanIN progression to PDAC to be less than 2% and taking up to 35 years or more, making screening of these lesions minimally beneficial even if feasible . Additionally, PanINs are found in nearly three-fourths of pancreatectomy and autopsy specimens by age 80, as well as in multiple individuals in their 20’s with low rates of progression to cancer.
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - “Conversely, another well-known precursor lesion in PDAC, intraductal papillary mucinous neoplasms (IPMNs), are estimated to be detectable in around 3%–6% of the general population and 10% of the population over 70, and can be monitored as compared to PanINs which cannot. Additionally, IPMNs demonstrate much higher progression rates to malignancy depending on the subtype, with rates as high as 90% in mixed-type types and as low as 3.3% in branch-duct types. However, there is difficulty in differentiating IPMNs that will progress to PDAC from those that will not, and further studies are needed to identify genetic differences, histological differences, or both to clearly stratify patients and avoid overtreating when unlikely to mitigate risk.”
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - For the 5-10% of diagnosed PDAC patients that have known genetic risk based on family history (defined as at least one first degree relative or two second-degree relatives diagnosed with pancreatic cancer in their lifetime) as well as an additional 3-5% of individuals that carry a predisposing genetic mutation (most commonly Hereditary Breast and Ovarian Cancer Syndrome (BRCA1/2), Fanconi Anemia, Familial Adenomatous Polyposis (FAP), Peutz-Jeghers Syndrome, Li-Fraumeni Syndrome, Familial Atypical Multiple Mole Melanoma Syndrome (FAMMM) or Lynch Syndrome (MLH1, MSH2/6 or PMS2 variants)), there is identified benefit from screening .”
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - In addition, analysis of ctDNA is much more specific than other blood-based biomarkers that are typically present at various levels as detection of any measurable amount of tumor DNA indicates the presence of malignancy. While studies provide evidence supporting ctDNA as a strong and even independent diagnostic marker, it is present in considerably smaller quantities in early disease which poses an obstacle to its use as an early diagnostic tool. However, its use alongside current biomarkers shows strong promise for improving precision in early diagnosis. A study by Cohen et al. in 2018 demonstrated that a combined assay combining both CA 19-9 and ctDNA abundance demonstrated superior precision over use of any biomarker alone as diagnostic tool for early PDAC, demonstrating the ability to detect disease in 60% of patients who had no traditional presenting symptoms of PDAC as well as 41% of PDAC patients who wouldbe diagnosed with Stage I disease.
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - Another significant issue is the biological variability inherent in PDAC tumors. Many patients may not shed detectable levels of ctDNA in addition to PDAC lacking a strong molecular profile outside of KRAS aberrations, which happens to be the most mutated oncogene in all human malignancies. With minimal amounts of tumor shedding in PDAC along with a limited molecular profile, ctDNA analysis may serve little to no benefit for individuals with nonspecific mutations or undetectable ctDNA. Additionally, with the majority of PDAC patients not having surgically resectable disease at diagnosis, it is unclear how disease properties and thus ctDNA are altered as disease progresses.
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - “While challenges remain in the field of ctDNA analysis for pancreatic adenocarcinoma, the potential for its advancement and use in clinical practice is vast. Continued evaluation of its potential and validity in large trials will be paramount for moving the benefits provided by liquid biopsy and circulating tumor DNA analysis to patients, paving the way for early diagnosis and more effective treatment strategies to ultimately improve patient outcomes for such an aggressive disease.”
Translating the multifaceted use of liquid biopsy to management of early disease in pancreatic adenocarcinoma.
Cox M, Vitello D, Chawla A.
Front Oncol. 2025 Mar 13;15:1520717. doi: 10.3389/fonc.2025.1520717. - Multicancer early detection (MCED) tests are an emerging technology for cancer screening. MCED tests can detect cancer signals from multiple cancers concurrently in biological samples such as blood, urine, saliva, or other bodily fluids. Some tests can suggest the most likely cancer origin, whereas others report cancer detected somewhere in the body. Although some MCED tests are currently commercially available, none are approved by the Food and Drug Administration or endorsed by any clinical practice guideline or recommendation. Most insurance companies do not currently cover MCED testing. MCED tests have not yet been evaluated for safety and effectiveness in randomized controlled trials. Because patients already are asking for MCED test prescriptions or for interpretation of results from tests acquired elsewhere, clinicians should be prepared to discuss what is known about the benefits, risks, and uncertainties of MCED testing, including performance characteristics in screening populations and preferred follow-up strategies for positive test results.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - Because patients already are asking for MCED test prescriptions or for interpretation of results from tests acquired elsewhere, clinicians should be prepared to discuss what is known about the benefits, risks, and uncertainties of MCED testing, including performance characteristics in screening populations and preferred follow-up strategies for positive test results. At this time, clinicians should not feel obligated to initiate discussions about MCED testing with their patients. However, clinicians should engage patients who inquire about getting tested or previous MCED test results in shared decision-making, and take the opportunity to offer and help patients complete age- and sex-appropriate guideline-recommended cancer screenings.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - Nearly half (1,016,900) of the cancer diagnoses and approximately three fifths (358,230) of the cancer deaths projected for 2025 are in cancers for which there are no recommended screening tests. Such cancers are commonly diagnosed at an advanced stage, when the prognosis is poor. Both the absence of screening strategies for these cancers and the suboptimal uptake and adherence for the few recommended screening tests, which target specific age ranges, lead to diagnosing only a minority of some of the most lethal cancers at a localized stage. The former category includes pancreatic (13.7%), ovarian (17.5%), esophageal (21.6%), and liver (40.8%) cancers, whereas the latter includes lung (25.8%) and colorectal (36.5%) cancers.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - There is also a paucity of data on conventional screening outcomes, including measures of sensitivity for any or specific cancers, true- and false-positive rates, indeterminate findings, incidental findings, and the range of potential harms, including complications from diagnostic procedures, overdiagnosis (finding cancers that will never cause symptoms), and overtreatment (unnecessary treatment for indolent cancers). The impacts of MCED testing on adherence to recommended screening and costs to patients, payers, as well as health care systems are also unknown. Finally, the performance of MCED tests varies between and within tests because companies use different diagnostic algorithms and sets of measured analytes, which are continuously being refined.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - The Exact Sciences CancerSEEK MCED test (now called Cancerguard), which is currently not commercially available to the public, was evaluated in the DETECT-A study, which screened 10,006 women aged 65–75 years with no history of cancer, enrolled from a population with high adherence to recommended screening.40 Abnormalities in baseline tests were confirmed with more rigorous blood testing; if the confirmatory testing was positive, then the participant underwent a total-body combined positron emission and computed tomography (PET-CT) scan because this MCED test did not report a CSO. Participants with an abnormal PET-CT scan were referred to specialists. Overall, 134 participants (1.34%) had a confirmed positive blood test, and 26 of these had a cancer diagnosed within 12 months of enrollment. A confirmed positive blood test (without a PET-CT scan) had a PPV of 19.4% (95% CI, 13.1%–27.1%) for cancer detection. Eight cancers (30.8%) were detected at stage I or II, including three cancers (uterine, ovarian, and thyroid) with no recommended screening tests.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - The PPVs for MCED tests are generally substantially higher than the PPVs for currently used single-cancer tests (e.g., mammography, stool blood tests, and low-dose CT scans) because the cancer prevalence is higher as a result of the aggregate detection of multiple cancers and because MCED test specificity is much higher than that seen with recommended screening tests. Given proof of efficacy, this would be a general benefit of multicancer detection relative to single-cancer testing. However, the tradeoff is that the sensitivity for detecting localized cancers is much lower than for recommended screening tests, and precancers cannot be detected. Notably, more than two thirds of the cancers diagnosed in the two prospective observational studies were diagnosed clinically or with recommended screening tests.
Multicancer early detection testing: Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - “Multicancer early detect We believe that clinicians are not obligated to initiate discussions on MCED testing until more is known about their effectiveness. Given the many uncertainties surrounding MCED testing at this time, patients who initiate a discussion about MCED testing should engage in a shared decision-making (SDM) process before undergoing testing. The goal of SDM is to inform a patient about the potential benefits, limitations, harms, and uncertainties around MCED testing to enable the patient to make decisions that align with their personal values and preferences. Factors to consider in ordering MCED tests include an individual’s reason for seeking an MCED test and their cancer risk factors, including age, sex, family history, and exposures, as well as life expectancy, and their willingness and ability to participate in the downstream evaluations of abnormal tests and, potentially, to undergo cancer treatment.”
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - “Patients should be informed about and agree to undergo diagnostic testing to evaluate a positive test result before embarking on MCED testing. A positive MCED test indicates that the patient is at a high risk for having cancer given the test’s high specificity. The ordering clinician, working in concert with health systems and specialist colleagues, is responsible for ensuring that a complete diagnostic evaluation is conducted. The follow-up of a positive MCED test depends on whether the test indicates a likely CSO. The predicted primary CSO reported by Galleri can be used to guide targeted evaluations toward a particular cancer, including imaging, laboratory testing, endoscopy, and, if indicated, tissue diagnoses. If that evaluation is unrevealing, a Galleri study protocol suggested that the next step could be either a targeted evaluation of the secondary predicted CSO or obtaining whole-body imaging.For tests that do not predict a cancer site of origin, such as the Exact Sciences multianalyte blood test, the manufacturer recommends total-body imaging with PET-CT scans.”
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - A negative workup could suggest that the initial blood test result was a false positive and the risk of cancer could be low, although the sensitivity of PET-CT scans as a second-stage screen for general cancers is unknown. The DETECT-A study reported that 95 of 98 participants with a false-positive result remained cancer free during a median 3.6 years of follow-up.However, a negative workup result could be misleading, particularly if the diagnostic evaluation was limited—or lacked the sensitivity to detect the cancer site of origin. This scenario could create enduring anxiety for many individuals. The next steps could be to repeat the MCED test, although the timing of subsequent testing after an apparent false-positive test result is uncertain, or to watchfully wait for symptoms. GRAIL offers a free retest at 3–6 months if the initial diagnostic evaluation of a cancer signal is negative.
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - Abnormal blood testing may lead to imaging studies, including PET-CT scans, which may detect one or more incidental findings in some patients. Primary care clinicians will need to use their clinical judgment, ideally supported by professional practice guidelines and guidance from multidisciplinary teams, to determine the next steps in the diagnostic workup. Diagnosing and treating incidental findings may be beneficial to patients if they are clinically significant. Conversely, findings may be clinically inconsequential or consequential without any potential for successful intervention, which may lead to complications from testing and treatments, anxiety, overdiagnosis, and additional costs, which all represent potential harms from any cancer screening, including MCED testing.
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - The gold standard study design for evaluating a screening program is an RCT with cancer mortality as an end point.28 One ongoing RCT is the NHS-Galleri study, which has collected blood from 140,000 asymptomatic participants in the United Kingdom.50 Half of the participants are being randomized to an intervention arm to have blood tested with an MCED test, and the other half are being randomized to the control arm to have blood stored. Intervention arm participants will be notified if a cancer signal is detected, and referred for further evaluation. Follow-up is expected to be for up to 4 years, and the primary, surrogate end point is the overall reduction in the incidence rates of stage III and IV cancers. Cancer-specific mortality is a secondary, delayed end point.
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - MCED testing represents a potential paradigm shift in cancer screening that provides an opportunity to detect numerous cancers with a single test. Although tests are commercially available, they are being evaluated in clinical trials, and data are not yet available to determine whether MCED testing for cancer screening meets many of the criteria that have been adopted and recognized as the standards for population screening.Because MCED testing is becoming increasingly available as new tests continue to be developed and evaluated,clinicians should be prepared to help patients make informed decisions about testing . Clinicians should further inform patients that MCED testing does not replace recommended cancer screening tests, and encourage them to make appropriate lifestyle changes to reduce cancer risk. MCED testing offers the potential to reduce the burden of suffering and premature death from cancer, although it will be some time before we know whether this potential can be realized. In the meantime, this article offers guidance to health care providers in their discussions with patients who inquire about MCED testing
Guidance for primary care discussions with patients.
Hoffman RM, Wolf AMD, Raoof S, et al.
Cancer. 2025 Apr 1;131(7):e35823. doi: 10.1002/cncr.35823. PMID: 40170549; - The Liquid Biopsy (LB) represents an ideal surrogate of tumor Tissue Biopsy (TB) when the aim is to obtain useful information on patient prognosis and personalized therapy. This technique renders it possible to isolate circulating tumor cells, circulating tumor DNA and other molecules from biological fluids. The most commonly used fluid for liquid biopsy is blood, but depending on the case it could be necessary to isolate the tumor components from other biological fluids such as urine, pleural effusion, cerebrospinal fluid, and others. The main advantages of liquid biopsy are the minimally invasive nature of the procedure and the possibility of analyzing all tumor clones. Limitations include difficulties in the isolation of tumor components and the requirement for highly sensitive analysis methods to avoid the risk of technical artifacts.
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343 - CTCs (circulating tumor cells) are tumor cells that detach early from the primary tumor or metastatic sites and circulate in the bloodstream before clinical or radiological signs of metastasis appear . Their half-life ranges from 1 to 4 hours before being destroyed by the immune system, although a fraction can escape destruction and spread to distant sites in the body. The concentration of CTCs in biological fluids depends on the type of tumor , but in general, it is low ( < 10 cells/mL of blood). For this reason, their identification is particularly challenginging and requires cell enrichment methods that use specific markers on other cell surface (e.g., epithelial adhesion proteins for positive selection or the CD45 marker for negative selection) or biophysical properties (such as cell size).
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343 - CtDNA is a highly fragmented, double-stranded DNA of 160–200 base pairs, with a half-life of about 114 minutes . While it has adequate sensitivity and specificity as a tumor biomarker, its proportion relative to ccfDNA varies widely, from < 0.1% to > 90% [40] . Factors influencing ctDNA levels include tumor characteristics and metastatic load (e.g., location, size, vascular infiltration, tumor status, and stage), with higher concentrations observed in patients with liver metastases and in lung and colorectal tumors. Additionally, it has been observed that advanced-stage tu- mors present higher ctDNA levels compared to localized tumors . The greater the tumor load , the greater the amount of ctDNA.”
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343 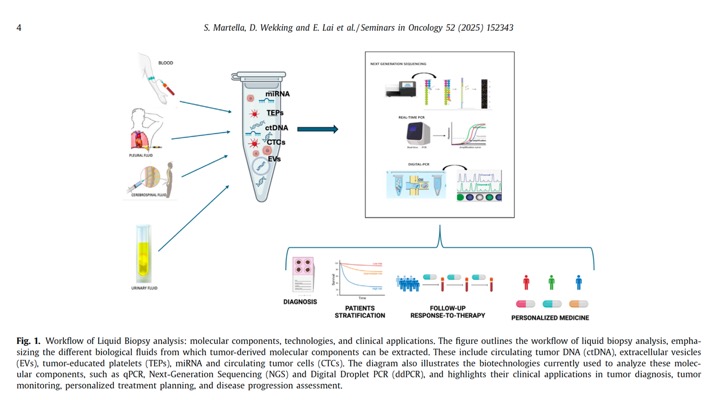
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343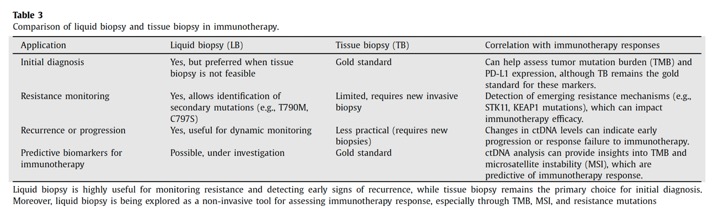
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343- Liquid biopsy represents an innovative opportunity for tumor monitoring and management, but there are still several gaps that limit its widespread use. One of the main issues is the lack of large-scale clinical studies. Most available data comes from pilot studies or small cohort studies, which, although useful for gathering preliminary information, are insufficient to validate liquid biopsy in broader clinical contexts. Therefore, it is crucial to conduct large-scale research involving more patients and diverse clinical situations to confirm the reliability and reproducibility of the results. Only through such studies will it be possible to develop comprehensive genomic databases capable of accurately tracking the molecular evolution of the tumor over time, thus improving the predictive capacity of liquid biopsy.
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Serafina Martella et al.
Seminars in Oncology 52 (2025) 152343 - However, despite advancements, liquid biopsy still has several limitations that hinder its widespread adoption: •Limited sensitivity and specificity: Current technologies are not always able to detect very low concentrations of ctDNA or identify biomarkers in patients with early-stage tumors, where ctDNA levels are low. •Difficulty in tumor localization: While liquid biopsy can identify the presence of tumors and mutations, it cannot always accurately localize the tumor or specify its primary origin. This limits its ability to assign biomarkers to specific tissues or organs. •Challenges in detecting tumor heterogeneity: Tumors are highly heterogeneous and contain different populations of cancer cells with varying mutations. Liquid biopsy may not capture this complexity, leading to an incomplete view of tumor biology. •Complex interpretation of analytical results: Data obtained from liquid biopsy can be challenging to interpret due to the variety of biomarkers involved and their variability between patients. The analysis of biomarkers must be performed with a very precise approach, considering comprehensive clinical data.
Liquid biopsy: An innovative tool in oncology. Where do we stand?
Seminars in Oncology 52 (2025) 152343
- We found that provider number of years in practice (DATA), awareness of challenges related to MCED testing (DATA), and perceived competence in MCED test use (DATA) were positively and significantly associated with receptivity to MCED test use in practice. An exploratory factor analysis extracted two components: receptivity to MCEDs and awareness of challenges. Surprisingly, these factors had a positive correlation (r = 0.124, p = 0.024). Providers’ perceived competence in using MCED tests and providers’ experience level were significantly associated with receptivity to MCED testing. While there was strong agreement with potential challenges to implementing MCEDs, PCPs were generally receptive to using MCEDs in cancer screening. Keeping PCPs updated on the evolving knowledge of MCEDs is likely critical to building receptivity to MCED testing.
Primary Care Provider Receptivity to Multi-Cancer Early Detection Test Use in Cancer Screening
Christopher V. Chambers et al
J. Pers. Med.2023, 13, 1673. https://doi.org/10.3390/jpm13121673 - We found that PCPs are generally receptive to the idea of incorporating MCED testing into their routine practice for cancer screening. This is in contrast to previous research that found that PCPs had concerns about potential problems associated with genetic testing as a screening tool. In particular, they reported insufficient confidence in their ability to order genetic testing and uncertainty around the clinical benefits of this testing as a screening method in low risk patients . In contrast with other genetic testing, MCEDs appear to have a more clearly defined place in the practice of primary care . MCED testing may represent a role for genetic testing for which PCPs can better understand the management of the results and their ability to explain them to their patients.
Primary Care Provider Receptivity to Multi-Cancer Early Detection Test Use in Cancer Screening
Christopher V. Chambers et al
J. Pers. Med.2023, 13, 1673. https://doi.org/10.3390/jpm13121673 - Not surprisingly, PCPs endorsed many of the items in the survey that related to potential challenges to the introduction of MCED testing into their practice. Several of these related to the amount of time that a discussion of MCED testing and the handling of the results would likely impose on an already busy patient schedule. Others related to concerns about the patient. These included whether patients would complete the additional testing associated with a positive test result and whether insurance would cover these recommended tests and procedures. Previous research has shown that patients often fail to complete recommended follow-up after a positive finding on conventional screening and that these delays may result in a new cancer diagnosis. The cost of the currently available MCED test alone will be outside the reach of many patients.
Primary Care Provider Receptivity to Multi-Cancer Early Detection Test Use in Cancer Screening
Christopher V. Chambers et al
J. Pers. Med.2023, 13, 1673. https://doi.org/10.3390/jpm13121673 - In summary, we found that PCPs in the study were generally receptive to the idea of incorporating MCED testing into their practice of screening for cancer. While they acknowledged the potential challenges to using MCED testing and the additional time that they would need to spend on ordering MCED testing and managing the results, the respondents signaled that they were receptive to MCED testing for cancer screening. Introducing MCED testing into routine screening for cancer will likely mean that the visits with the PCP will take longer or that other trained staff will need to be involvedin the patient education process.
Primary Care Provider Receptivity to Multi-Cancer Early Detection Test Use in Cancer Screening
Christopher V. Chambers et al
J. Pers. Med.2023, 13, 1673. https://doi.org/10.3390/jpm13121673
- Background Emerging blood-based multi-cancer early detection (MCED) tests can detect a variety of cancer types across stages with a range of sensitivity, specificity, and ability to predict the origin of the cancer signal. However, little is known about the general US population’s preferences for MCED tests.
Objective To quantify preferences for MCED tests among US adults aged 50–80 years using a discrete choice experiment (DCE).
Conclusions While there is significant heterogeneity in cancer screening preferences, the majority of participants preferred MCED screening and the accuracy of these tests is important. While the majority of participants preferred adding an MCED test to complement current cancer screenings, the latent class analyses identified a small (16%) and specific subset of individuals who value attributes differently, with particular concern regarding false-negative and false-positive test results, who are significantly less likely to opt-in.
Patient Preferences for Multi‑Cancer Early Detection (MCED) Screening Tests
Heather Gelhorn et al.
The Patient - Patient-Centered Outcomes Research https://doi.org/10.1007/s40271-022-00589-5 - “Offering an MCED screening test as part of the standard of care to individuals between the ages of 50 and 80 years is likely to be well received by the majority of this population. Based on the results of the current study, this could represent a viable approach to population-based cancer screening.”
Patient Preferences for Multi‑Cancer Early Detection (MCED) Screening Tests
Heather Gelhorn et al.
The Patient - Patient-Centered Outcomes Research https://doi.org/10.1007/s40271-022-00589-5 - "Recent advances in Artificial Intelligence (AI) indicate that AI has the potential to enhance how cancer is studied, diagnosed, and treated. In the near future, AI may be able to predict certain clinical outcomes, such as a patient’s response to anti-cancer drugs or combinations of such drugs. Analysis of large datasets using AI may also help discover novel cancer mechanisms, novel biomarkers of therapy response, or uncover novel therapeutic targets in cancer models and cancer patients . For example, tumor cells can be imaged directly in tissue or after being cultured and treated with pharmacological agents, then analyzed using deep-learning tools to unearth features associated with drug response or disease processes such as metastasis. Automatically integrating several disparate data types obtained from patient data, such as radiology images and molecular profiles of blood, may allow AI to improve patient diagnoses and detect cancer earlier than currently possible.”
Artificial intelligence in oncology: From bench to clinic
Jamal Elkhader, Olivier Elemento
Seminars in Cancer Biology 84 (2022) 113–128 - "There are however numerous challenges in developing accurate AI models and implementing them in the research and/or clinical setting. The datasets used to train AI models have started falling under more scrutiny than before as implicit biases in some training datasets have become apparent, the most important of which being lack of ethnic diversity and under-representation of certain groups, e.g. African Americans. Another related challenge is the relative scarcity of datasets that can be used as external validation of AI models, owing to privacy concerns and competition between medical centers that limit data sharing; this in turn hampers the vetting necessary to adopt AI models in clinical environments. Here we review recent advances and opportunities in AI and data science applied to cancer and the challenges AI will need to overcome to thrive in the laboratory and the clinic.”
Artificial intelligence in oncology: From bench to clinic
Jamal Elkhader, Olivier Elemento
Seminars in Cancer Biology 84 (2022) 113–128 - “Machine learning, and especially deep learning, have been used to dramatically enhance research in field of digital pathology. One application of deep learning is the detection and classification of specific cell types in histopathology slides. For example, Sirinukunwattana et al. proposed a deep learning method based on spatially constrained convolutional neural network for detecting and classifying cell nuclei in colon cancer tissue. Qupath, an open-sourced software for digital pathology and whole slide image analysis is capable of detecting nuclei but also comes with a user interface to label histopathology slides and create datasets for training new machine learning models. Within histological slides, deep learning can also be used to detect certain areas of interest and classify specific types of lesions. ”
Artificial intelligence in oncology: From bench to clinic
Jamal Elkhader, Olivier Elemento
Seminars in Cancer Biology 84 (2022) 113–128 - Just like pathology, deep learning is dramatically enhancing the field of radiology. To address the detection and treatment of lung cancer, Hua et al. used a deep learning framework to perform pulmonary nodule classification using CT images from the Lung Image Database Consortium dataset In the case of head and neck cancer, the identification of tumor extranodal extension is known to be difficult to diagnose radiographically, and has previously been diagnosed by postoperative pathology. Kann et al. used a CNN to identify tumor extranodal extension and nodal metastasis, trained on >2000 CT-segmented lymph node samples from patients at the Yale School of Medicine, and tested on 131 samples, achieving an AUC of 0.91 (CI: 0.85 0.97) on both extranodal extension prediction and nodal metastasis prediction. With a >85 % accuracy (extranodal extension: PPV: 0.66, NPV: 0.95; nodal metastasis: PPV: 0.88; 0.82), the use of this model as an identification tool shows favorable potential . ”
Artificial intelligence in oncology: From bench to clinic
Jamal Elkhader, Olivier Elemento
Seminars in Cancer Biology 84 (2022) 113–128 - “Artificial intelligence will continue to play a key role in various facets of the medical field, including drug discovery and clinical decision-making. Implementing artificial intelligence techniques into clinical practice is a promising area, allowing for progress to be made while remaining both vigorous and transparent. With improved imagine diagnostics, the efficient utilization of imaging, molecular, and cellular cancer data to predict clinical outcomes, and providing a catalyst for the development of oncologic drugs, AI has the potential for a powerful transformation. The influx of medical data is likely to continue to blossom as precision medicine continues to be implemented."
Artificial intelligence in oncology: From bench to clinic
Jamal Elkhader, Olivier Elemento
Seminars in Cancer Biology 84 (2022) 113–128







