Short and Long Term Complications of Endovascular Stents: MDCT Findings and the Importance of Volumetric Visualization with 2D MPRs and 3D Rendering for Detection and CharacterizationShort and Long Term Complications of Endovascular Stents: MDCT Findings and the Importance of Volumetric Visualization with 2D MPRs and 3D Rendering for Detection and Characterization Elliot K. Fishman, MD, FACR |
Type I Endoleak
|
Type I Endoleak 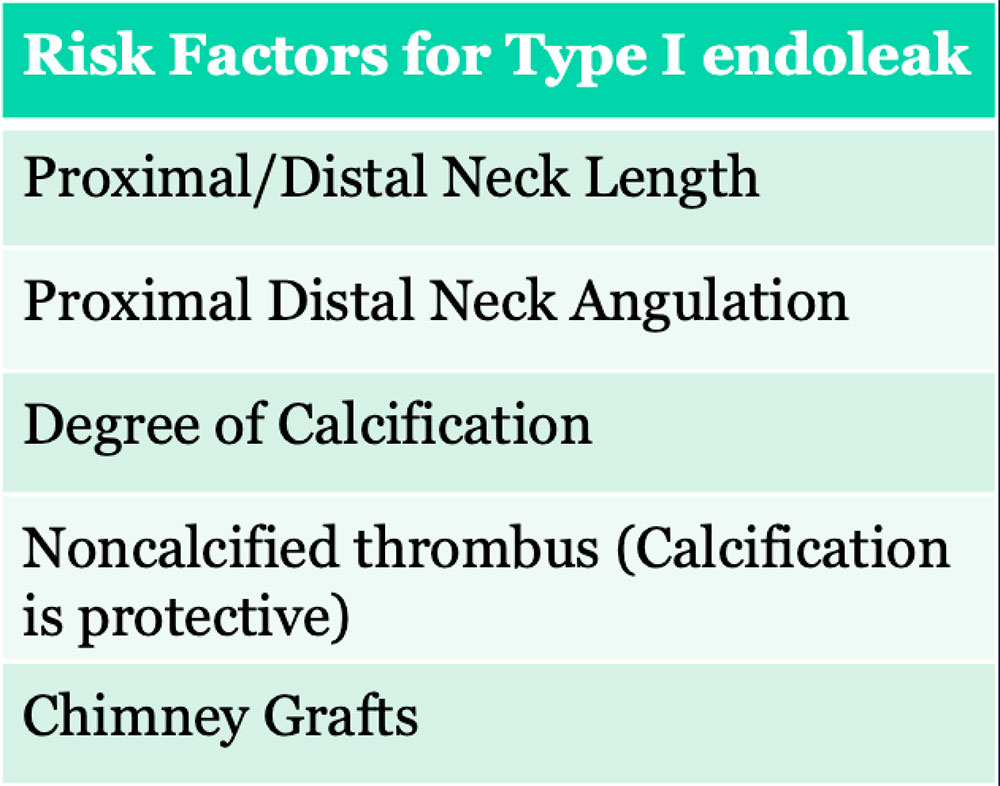 |
Type I Endoleak 60 year old woman with EVAR presented to ED with pain. Coronal IV CECT images show a new Type I endoleak (red arrows) and stent deformity (yellow arrows) in setting of infection (see slide 24) 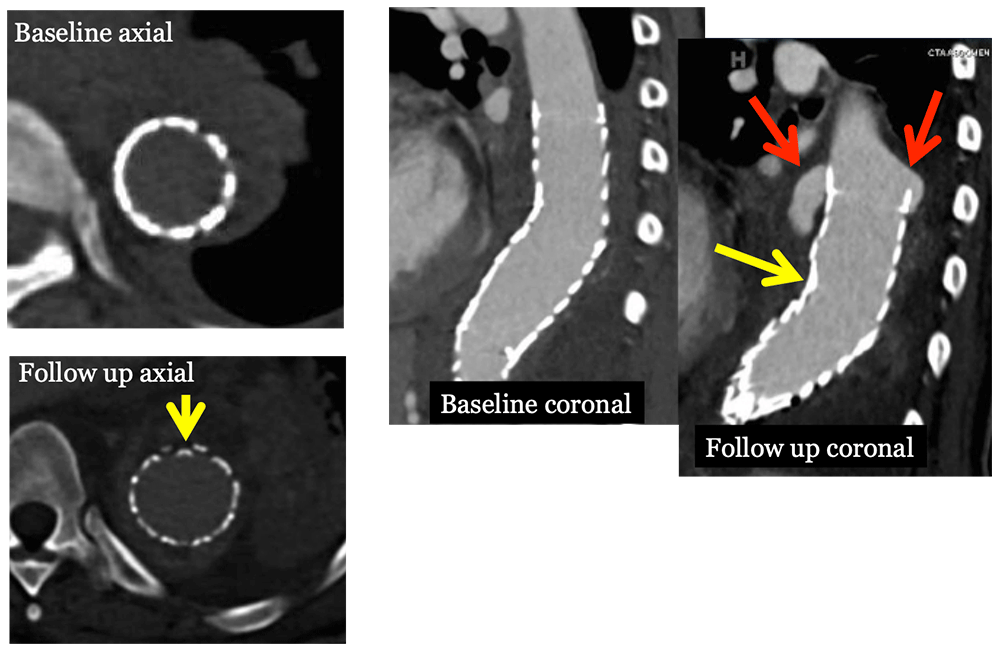 |
Type II Endoleak
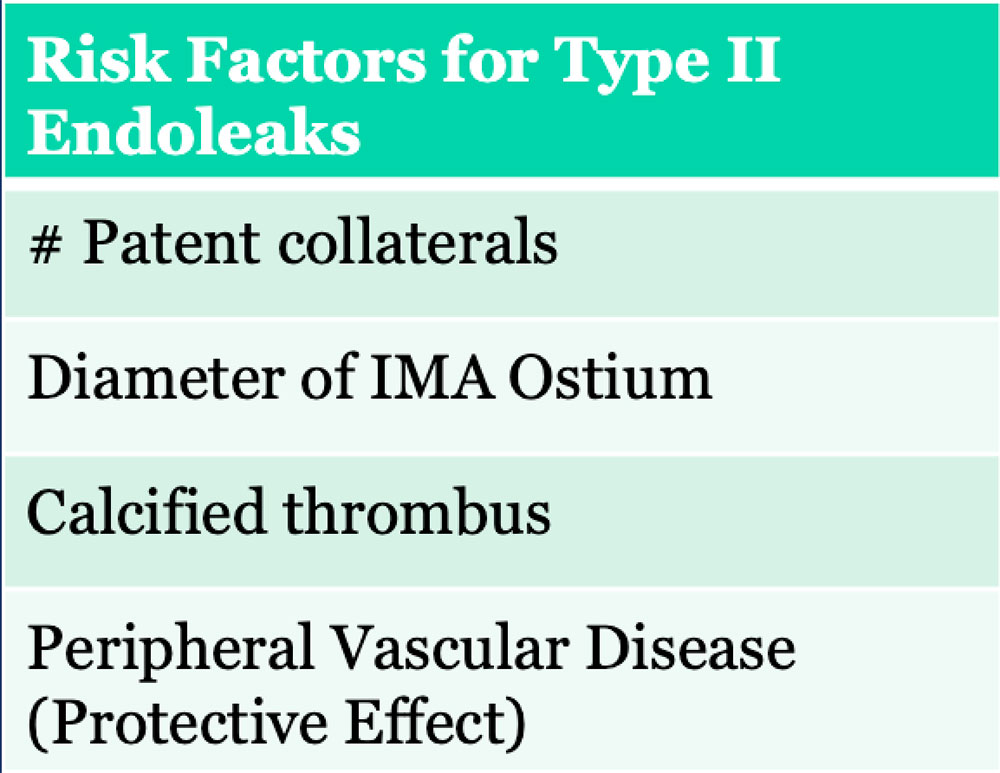 |
Type II Endoleak Small type II endoleak (circle) in anterior sac. Search for feeding vessel, which is typically the inferior mesenteric artery (arrow) with anterior endoleaks. 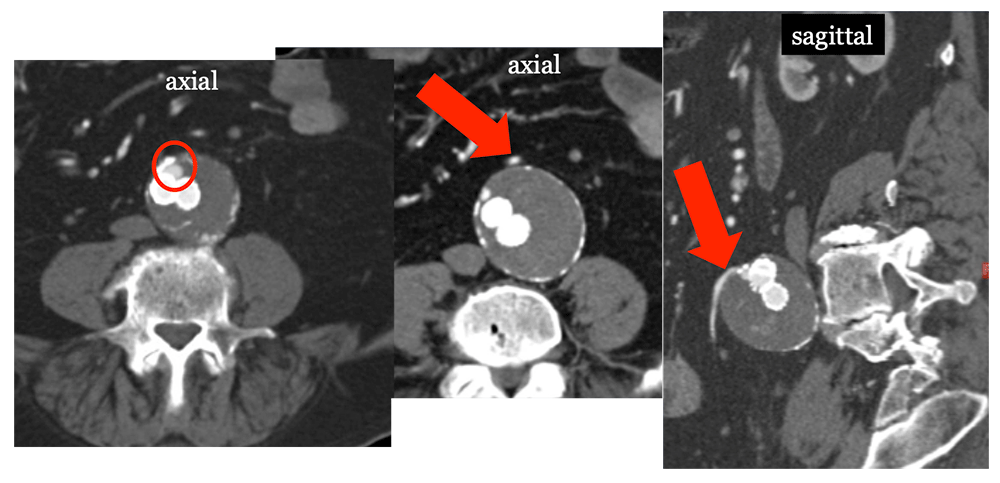 |
Type III Endoleak
|
Type III Endoleak 75 Year old male with history of EVAR presenting with back pain. MDCT shows kinked stent graft (yellow arrow) causing type III endoleak (circle), which resulted in rupture (red arrow). 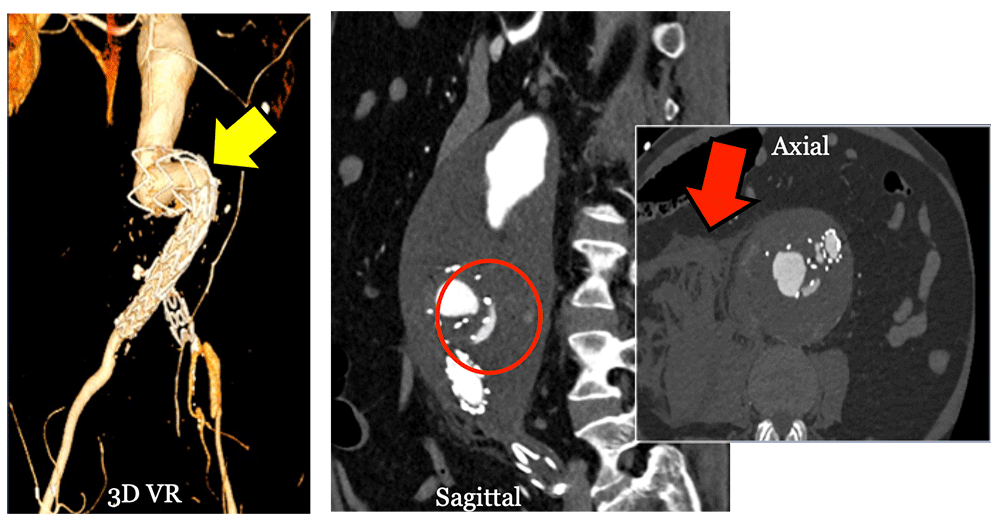 |
Type IV Endoleak
|
Type V Endoleak
|
Migration and Kinking
|
Dissociation of components 77 year old male s/p EVAR for AAA. Routine IV CECT at 1 year follow up revealed stent graft dissociation (yellow arrows), aneurysm sac increase (circle) to 10 cm and large type III leak (E) in the absence of symptoms, probably sequela of recent fall. 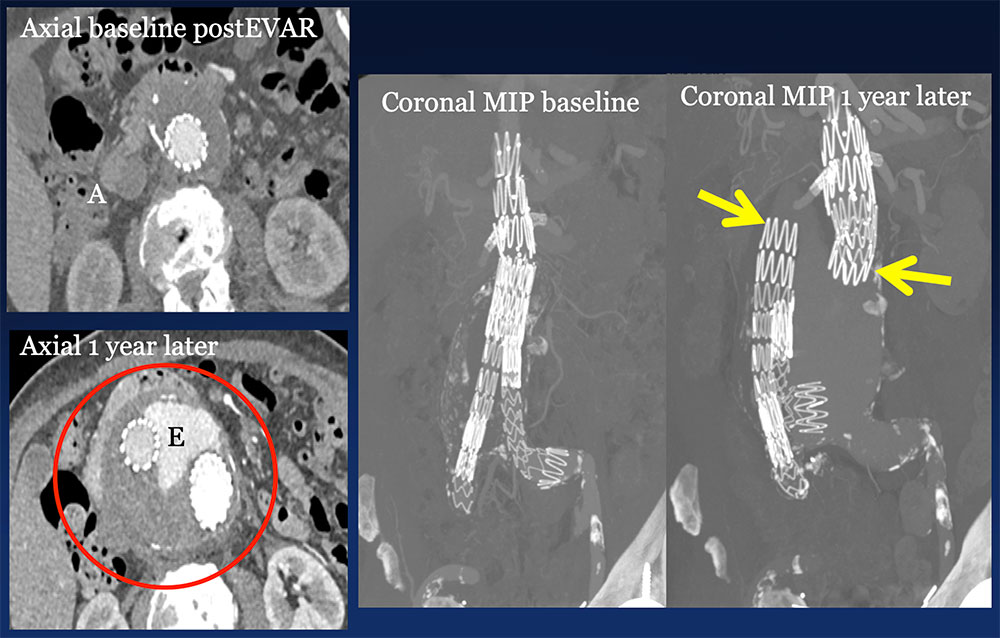 |
Thrombosis, Occlusion and Stenosis
|
Infection
|
Infected EVAR and Type I Endoleak 60 year old woman with EVAR presented to ED with pain (same patient as slide 14). Coronal IV CECT images show a new Type I endoleak (red arrows) in the setting of lung infection. Note infected left lower lobe (L), left pleural effusion (P) and small gas bubbles (yellow arrows). 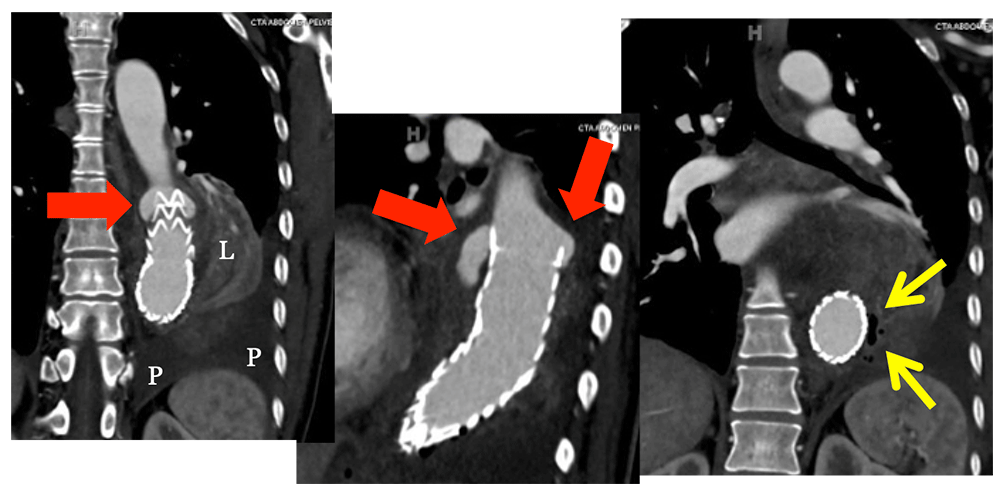 |
Aortoenteric Fistula
|
Aortoduodenal Fistula Patient with fractured stent (yellow arrow) status post replacement stent. Two years later, the patient presented to ED with melena due to aortoduodenal fistula from the aneurysm sac (red arrows). 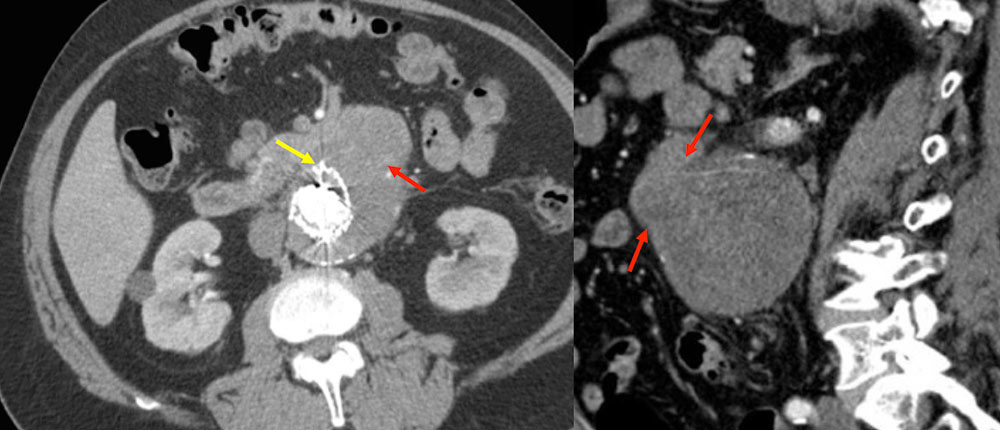 |
Aortoduodenal Fistula 87 year old female with CAD, AAA s/p endoluminal repair and revision who presented with progressive weakness. She was found to be severely anemic with baseline hemoglobin of 6 due to fistula between aneurysm sac and adjacent duodenum. In this case, gas bubbles (red arrows) are identified in the aneurysm sac, confirming fistulous connection (yellow arrows) to the adjacent duodenum. Heterogeneity within the sac reflects hematoma due to sac rupture. 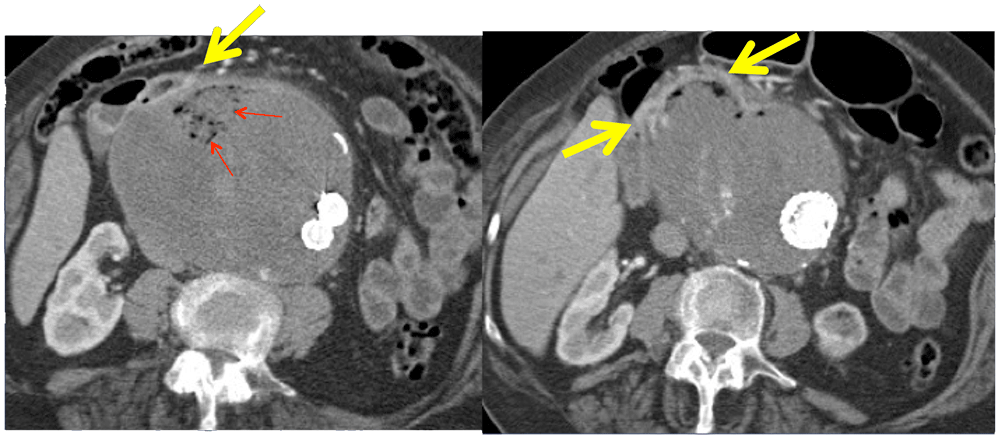 |
Aortocolonic Fistula 78 year old male s/p aortoiliac EVAR with chronic occlusion of left limb and chronic endoleak who presented with LGIB. CECT revealed aortocolonic fistula (arrows) from transverse colon to anterior sac. 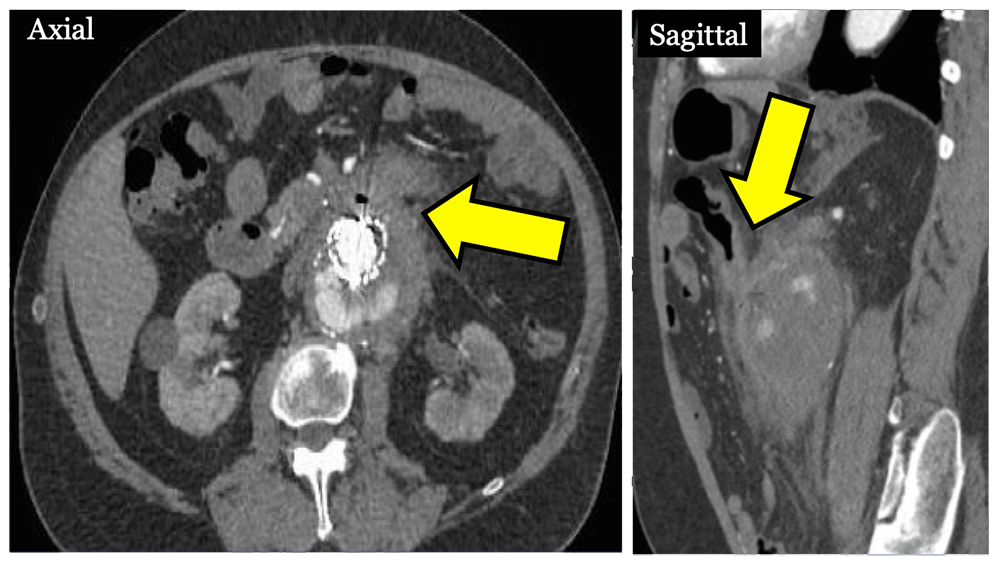 |
Retrograde Aortic Dissection
|
TEVAR with Retrograde Dissection 82 year old female status post aortic arch and descending thoracic EVAR who presented with cardiac arrest due to ruptured retrograde Type A dissection (circle). Note extensive mediastinal hematoma (H) markedly narrowing the pulmonary arteries. This is a potential complication of a stent positioned in the aortic arch. 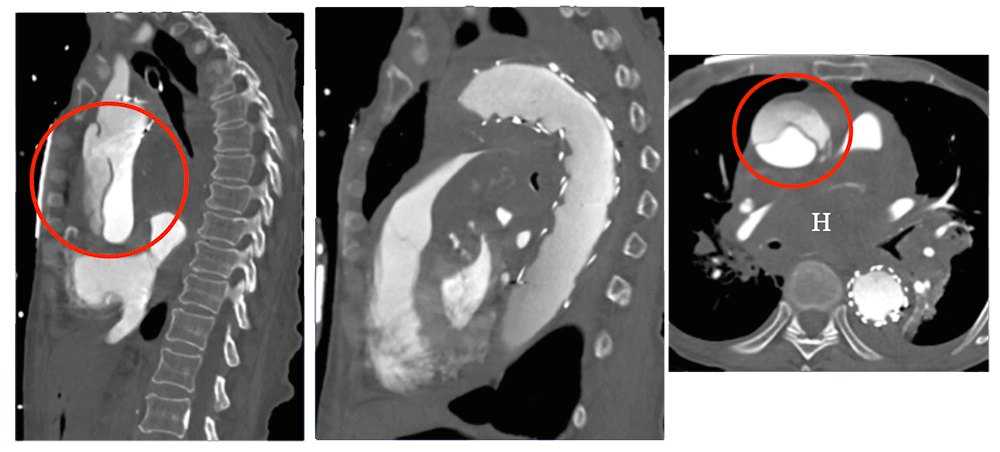 |
Rupture
|
Ruptured Aneurysm Sac Post AAA EVAR with endoleak seen best on venous phase (large arrow), which led to aneurysm sac rupture (small arrows). 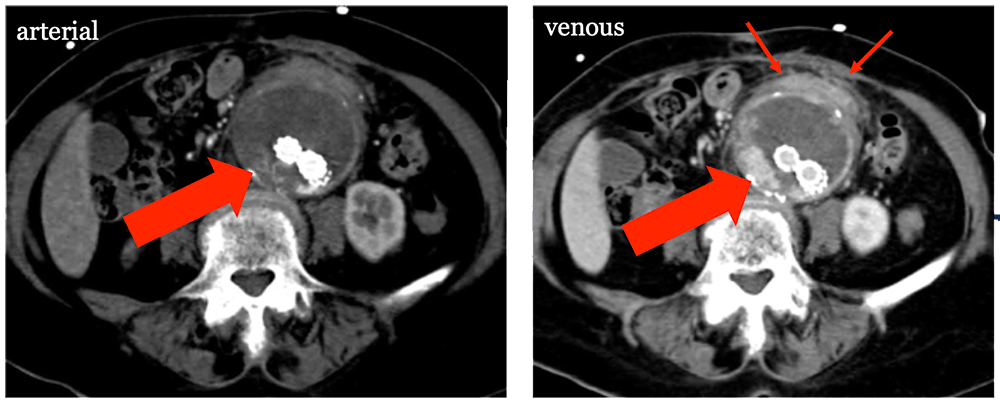 |
References
|
References
|
References
|
References
|
References
|
