Multi-modality Imaging of Liver Transplant: What you cannot afford to miss
Multi-modality Imaging of Liver Transplant: What you cannot afford to miss Elliot K. Fishman M.D. Johns Hopkins Hospital |
Objectives
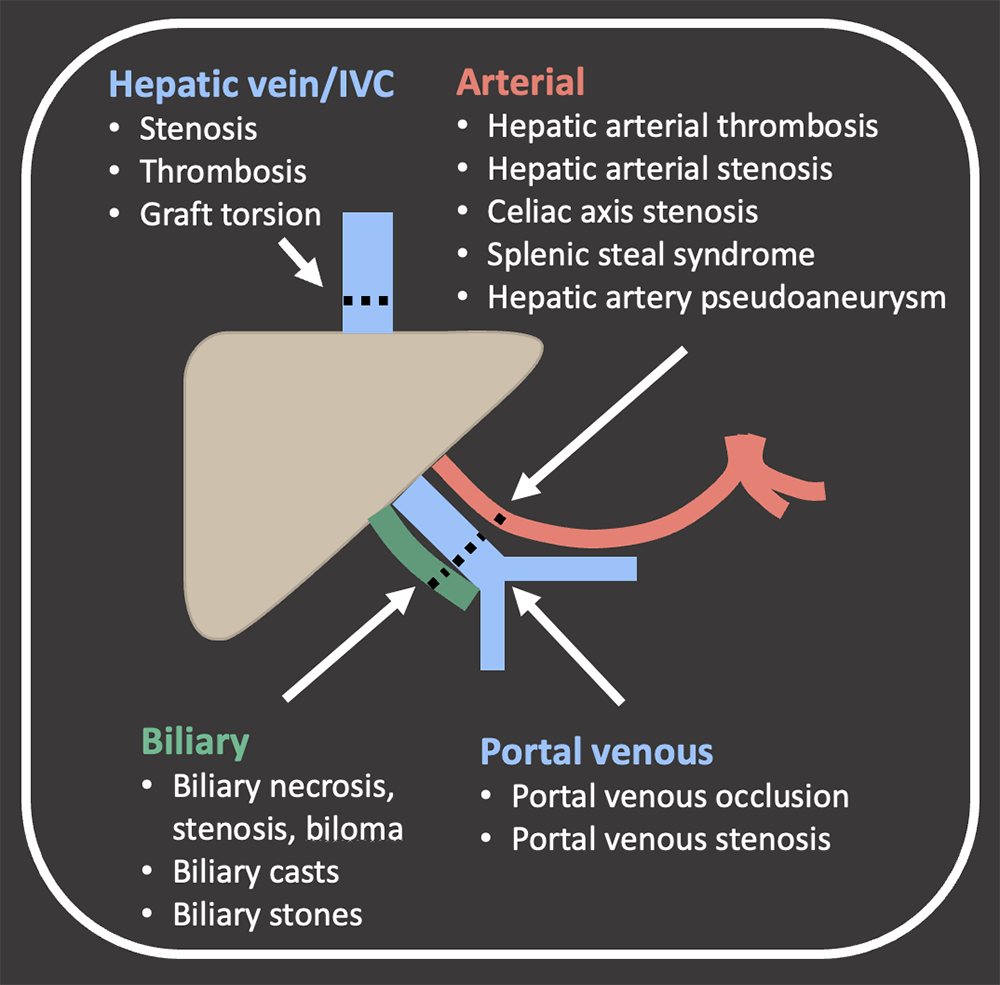 |
Post-transplant Ultrasound Assessment Practical Points
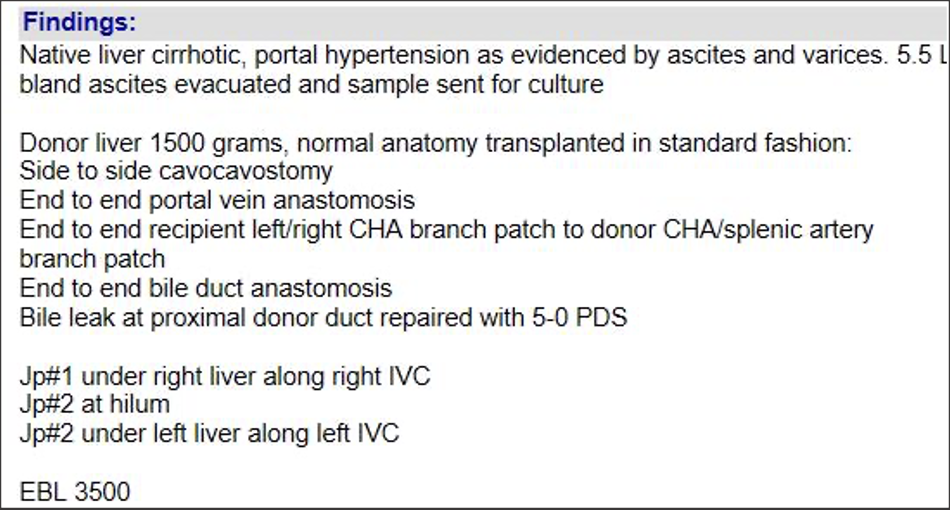 |
Hepatic Artery Normal Appearance in OLT Surgical anastomosis:
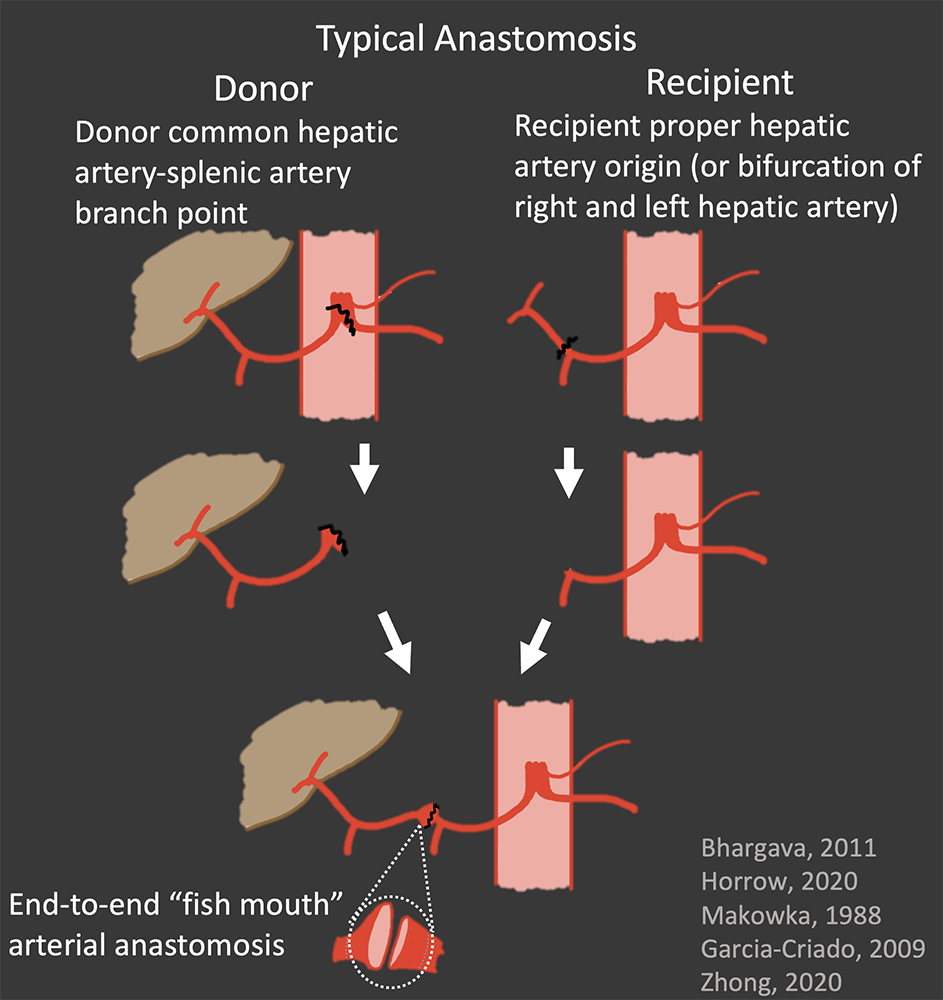 |
Hepatic Artery Normal Appearance in OLT Surgical anastomosis:
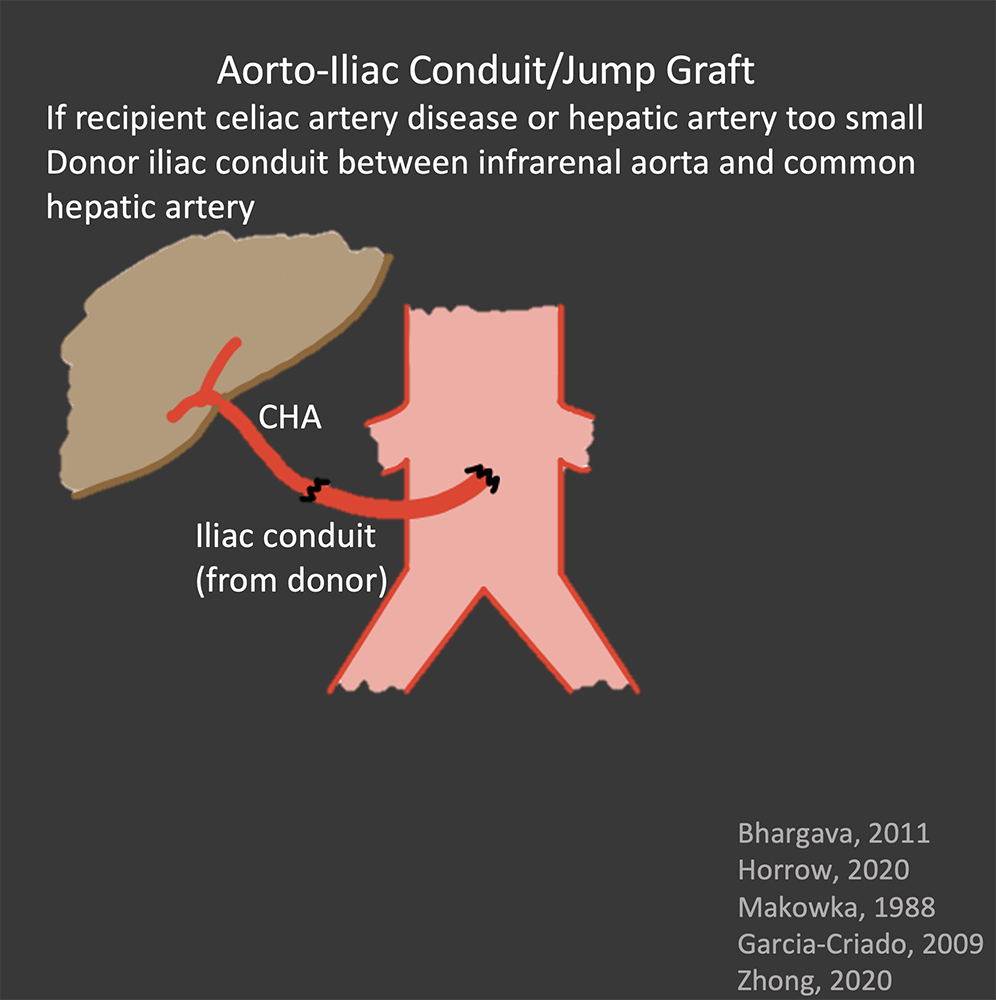 |
Hepatic Artery Normal Appearance in OLT Surgical anastomosis:
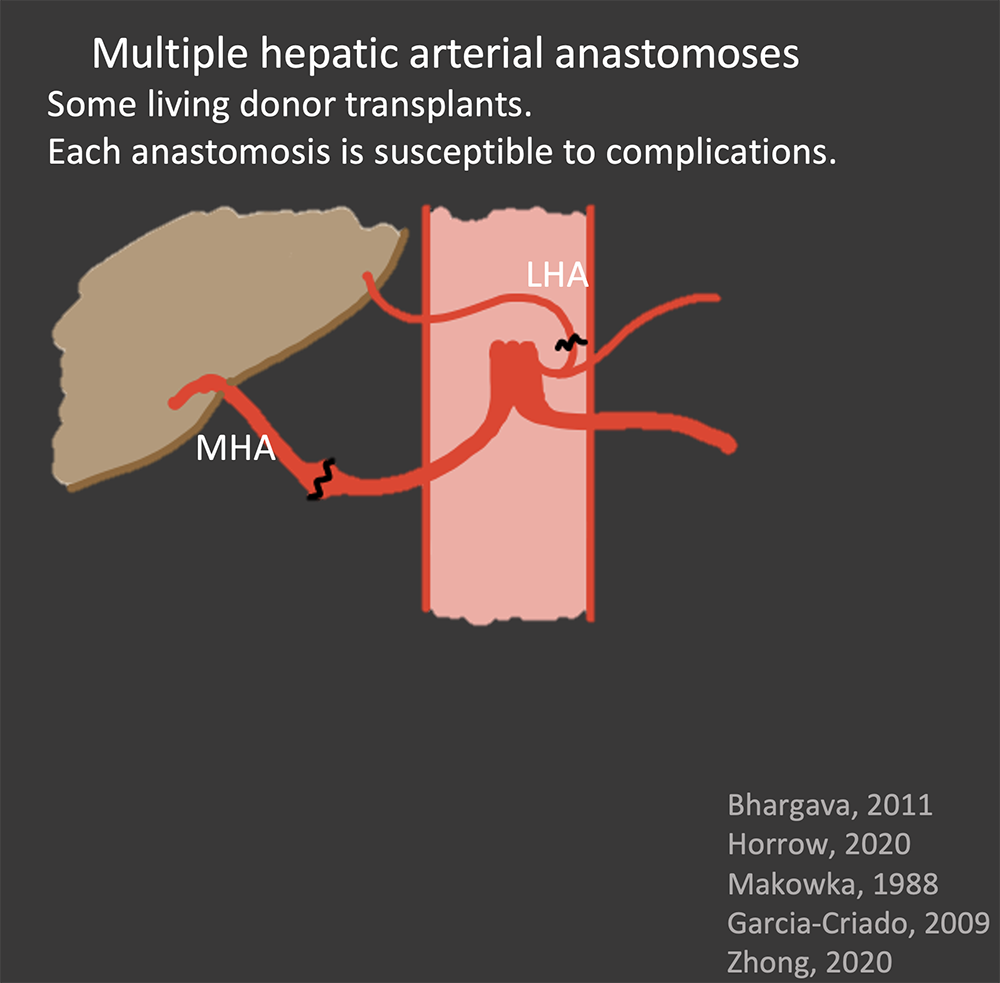 |
Case: Transient Elevated RI Transient Elevated RI (>0.8) is a common benign finding in the first 4 days post-transplant. RIs should be followed until normalization. Contrast-enhanced ultrasound is excellent to confirm patency of the hepatic artery and may be performed at the bedside. There is emerging data on the use of Superb Microvascular Imaging ultrasound technique to evaluate low-flow vessels. Garcia-Criado, 2009; Güven, 2019 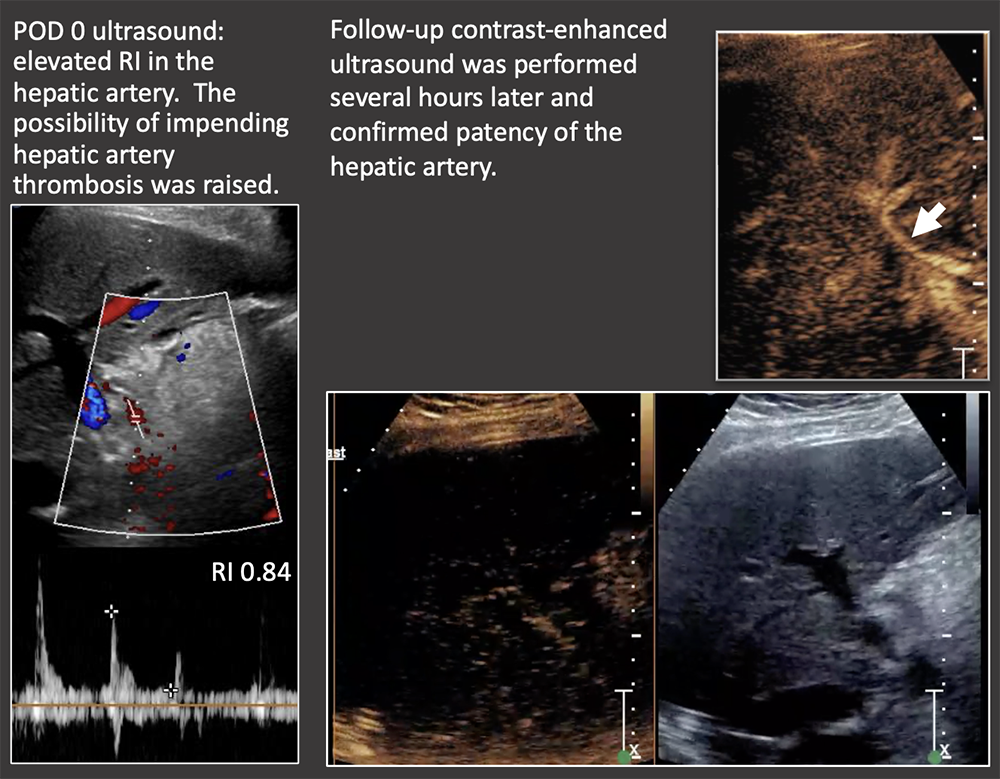 |
Case: Early Hepatic Artery Thrombosis Clinical follow-up: On post-op day 14, patient underwent new liver transplant. The liver explant demonstrated hepatic artery thrombosis with ischemic injury and necrosis. Hepatic artery thrombosis is the most common vascular complication. Loss of flow on ultrasound is diagnostic of an hepatic artery thrombosis. Doppler US has nearly 100% sensitivity for early (<1 week post-op) hepatic artery thrombosis. There is a progression of ultrasound findings in impending hepatic arterial thrombosis: decreased diastolic flow (elevated RI). → blunting of systolic upstroke and parvus tardus wave form (decreased RI) → loss of flow Bhargava, 2011; Sannananja, 2018; Horrow, 2007, ; Vaidya, 2007 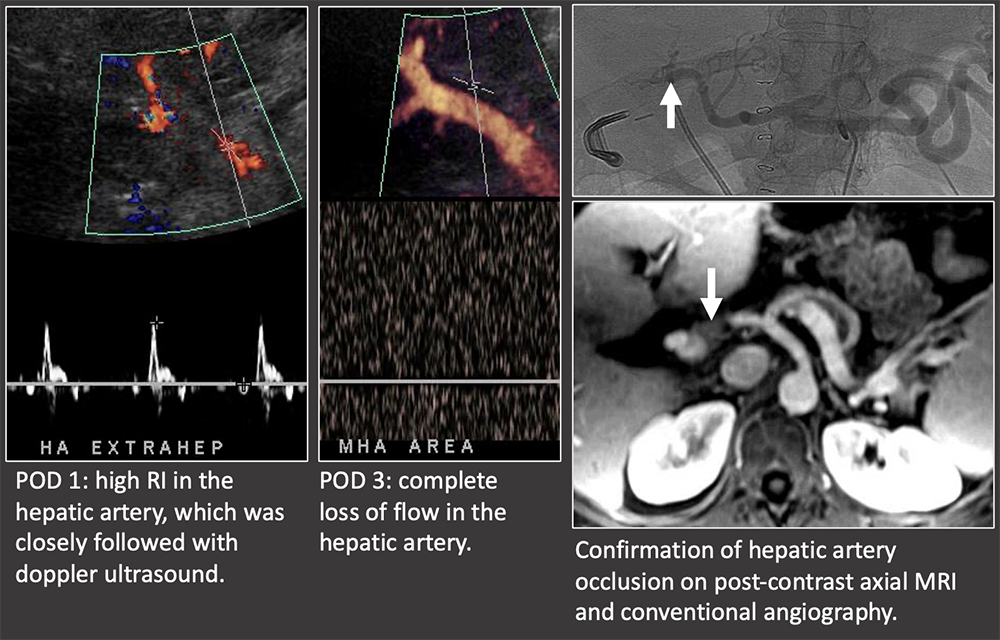 |
Case: Early Hepatic Artery Thrombosis 2 weeks post-op: Markedly elevated liver function tests found on routine follow-up. US shows abnormal low RI in the HA at the porta hepatis, no flow more centrally and focal hepatic infarct. CT confirms HA thrombosis and hepatic infarct. Clinical follow-up: Patient required repeat OLT 4 days later. Pathology of the liver explant showed massive panlobular hepatocyte necrosis and bile duct loss consistent with hepatic artery occlusion. Biliary ischemia is a primary concern in hepatic artery disease because the bile ducts are solely supplied by the arterial system. Complications can be bilomas, biliary leaks, and biliary strictures. There are variable complications after early hepatic arterial thrombosis, ranging from fulminant hepatic necrosis to self-limiting complications. Hepatic infarcts can be readily visualized on ultrasound. Bhargava, 2011; Sannananja, 2018; Horrow, 2007, ; Vaidya, 2007 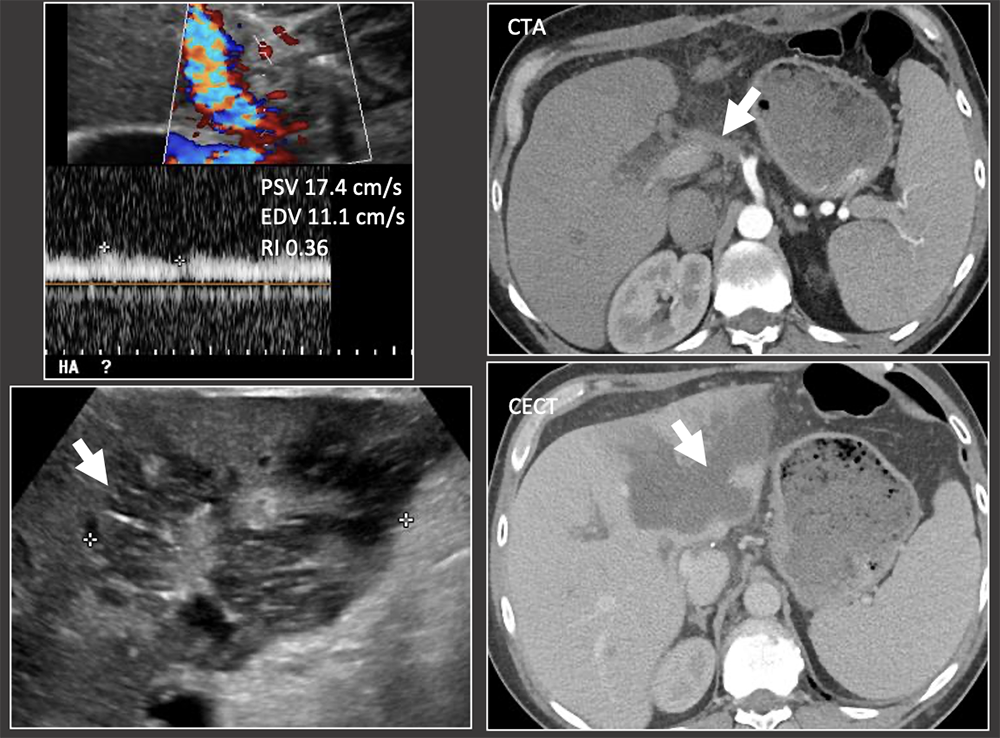 |
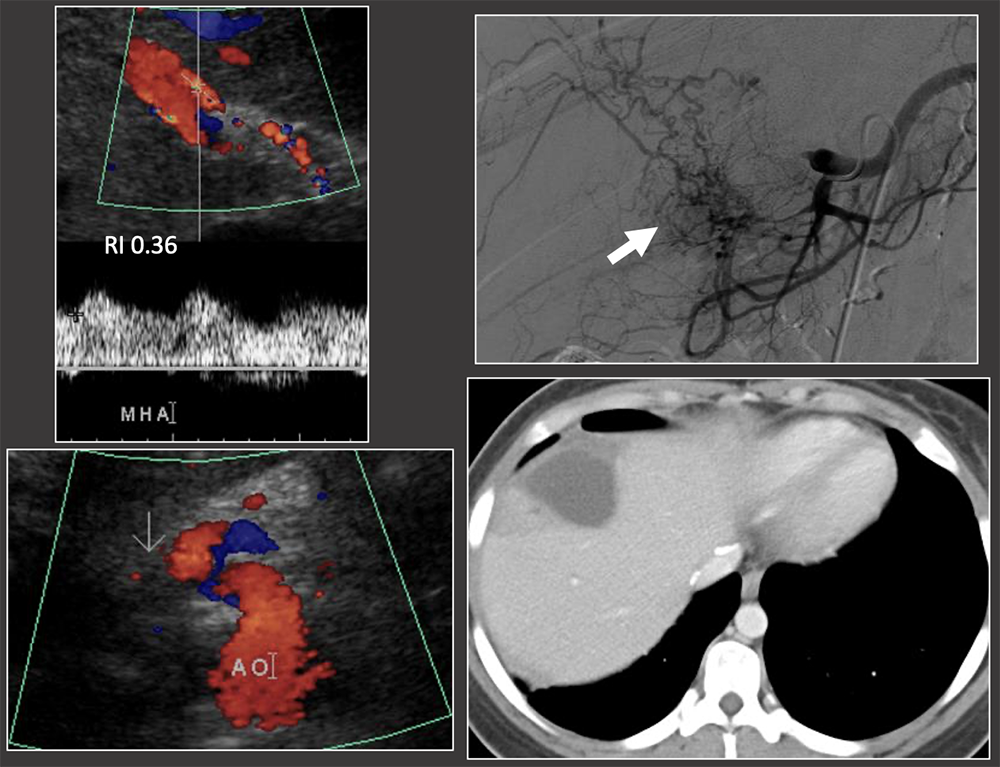
Case: Late Hepatic Artery Thrombosis Clinical follow-up: graft survived with recurrent liver abscesses for 6 years. Rising liver function tests may be the first indication that something is wrong with the hepatic graft. Bhargava, 2011; Sannananja, 2018; Horrow, 2007, ; Vaidya, 2007 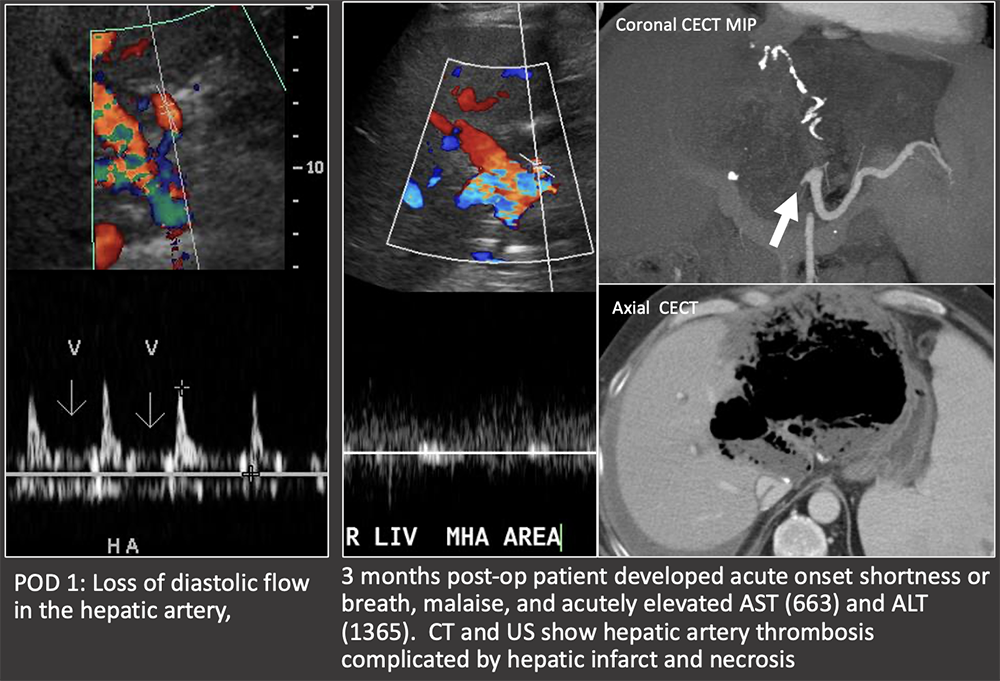 |
Case: Late Hepatic Artery Thrombosis 1 month after liver transplant: low RI in hepatic artery although arterial flow is present. Extensive collateral arteries on angiography. Hepatic infarct seen on CT. Ultrasound is less sensitive for the detection of late hepatic arterial thrombosis (>1 week post-op) due to the formation of collateral vessels. Outcomes of late hepatic arterial thrombosis are not as severe as early thrombosis because of the formation of collateral vessels. Differential diagnosis of low RI:
 |
Case: Hepatic Artery Thrombosis Intrahepatic collection on CT and US. RI in the hepatic artery is normal. Hepatic artery occlusion on angiogram. Intrahepatic collections may be due to underlying hepatic artery thrombosis, even in the setting of normal hepatic artery RI, because RI may be preserved in late hepatic artery thrombosis. Bhargava, 2011; Sannananja, 2018; Horrow, 2007, ; Vaidya, 2007 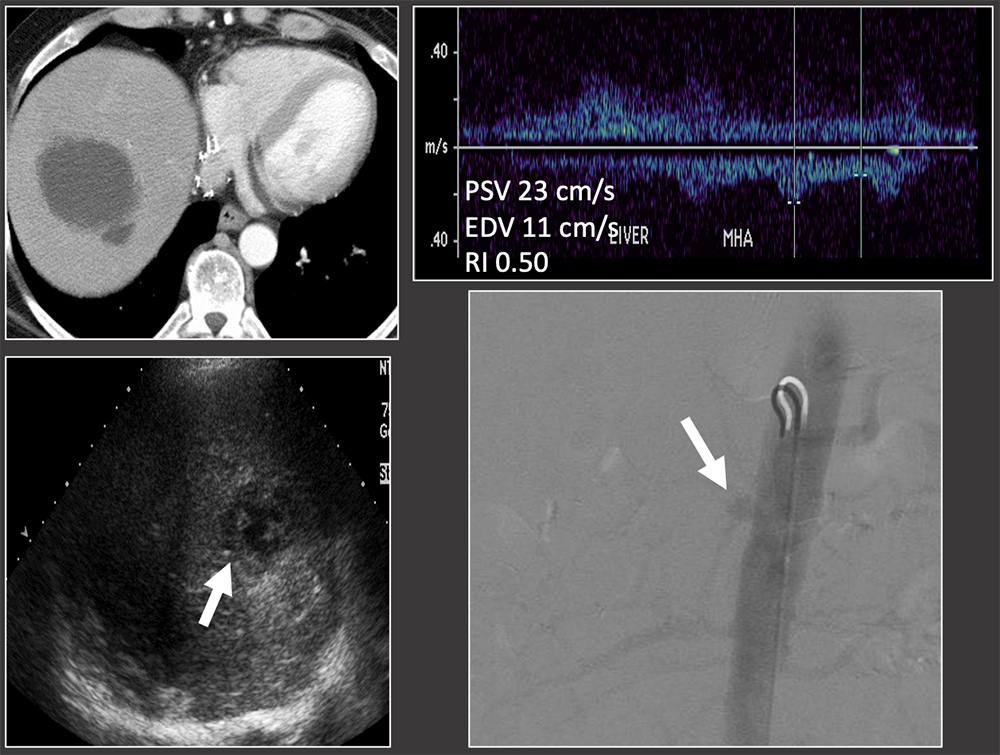 |
Case: Hepatic Artery Stenosis Low RI necessitates evaluation of the hepatic artery to look for a more proximal hepatic artery stenosis or thrombosis. US findings:
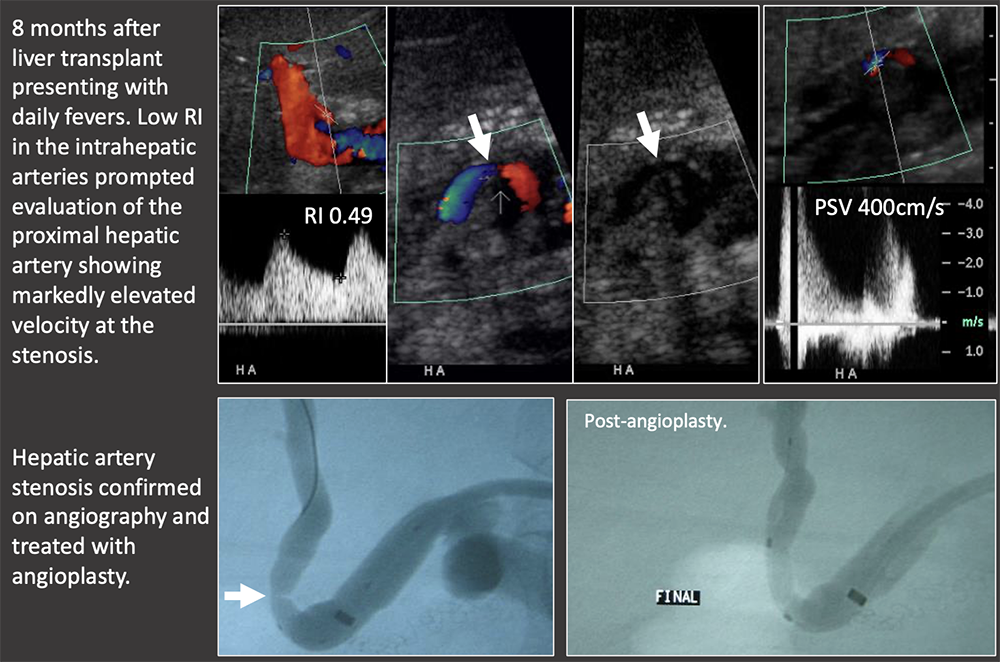 |
Case: Hepatic Artery Stenosis Severe hepatic artery stenosis may cause graft ischemia, although most mild stenosis only causes mild graft dysfunction. If there is a low RI in the hepatic artery, but the proximal artery cannot be followed with ultrasound, then it must be evaluated on another modality. Differential diagnosis of low RI in the intrahepatic arteries:
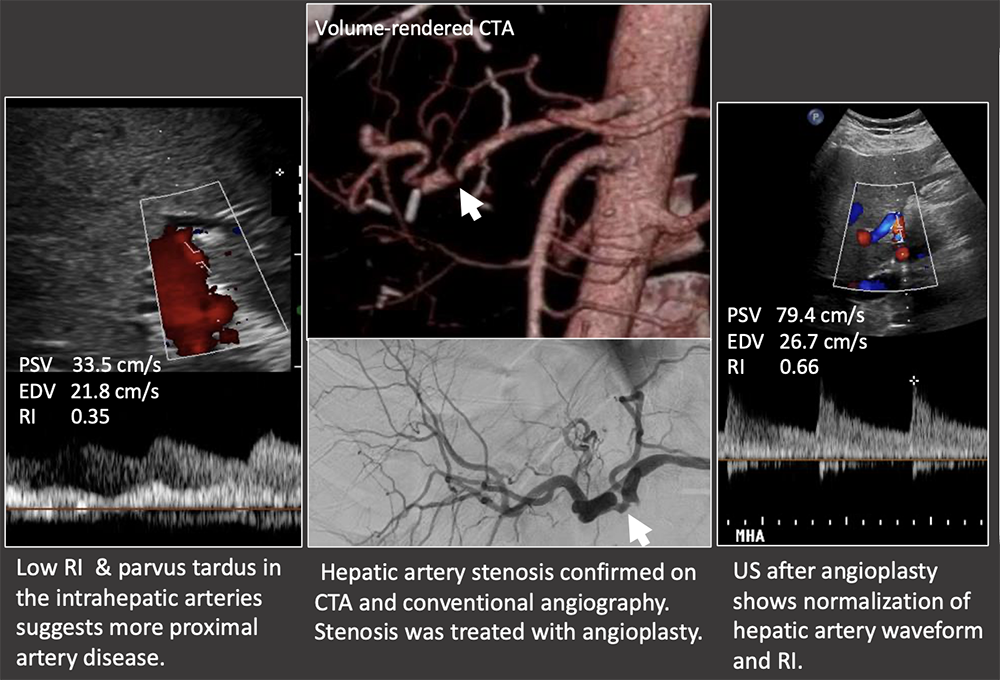 |
Case: Celiac Artery Stenosis Celiac axis stenosis may be graft-threatening if not recognized pre-operatively. The celiac artery, SMA, and variant arterial anatomy should always be addressed in the pre-transplant imaging exam. Celiac axis stenosis is often asymptomatic pre-operatively because of retrograde collateral flow from the SMA. After transplant, the graft is entirely dependent on arterial flow from the celiac axis because of surgical division of collaterals. Suspect celiac artery stenosis if the post-operative RI is decreased but there is no hepatic arterial thrombosis or stenosis. Horrow, 2020 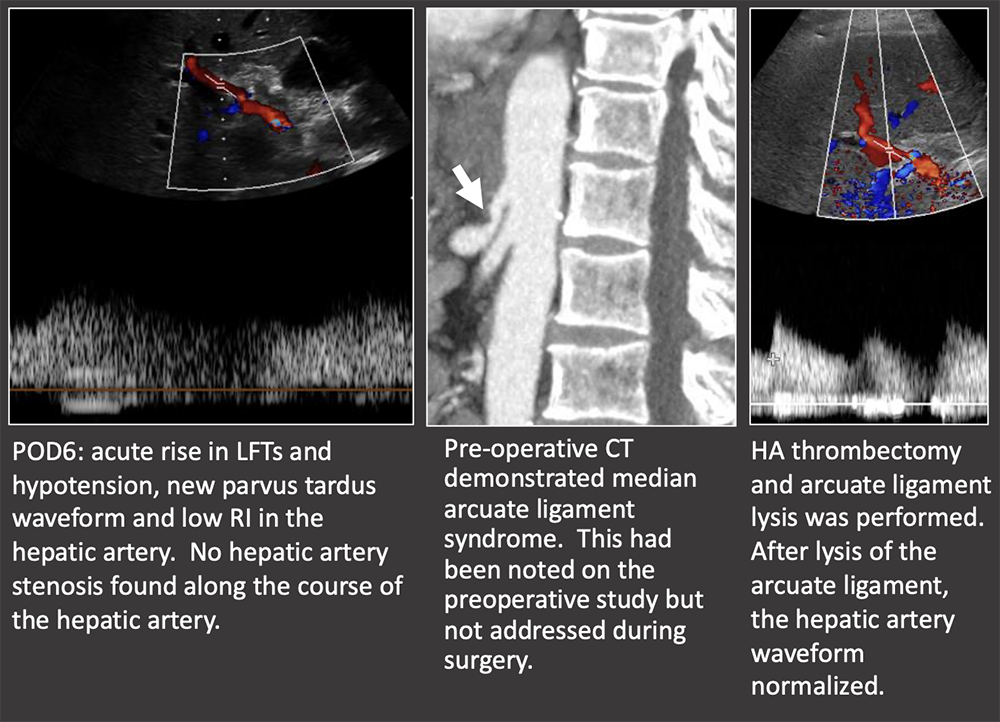 |
Case: Splenic Steal Syndrome Splenic steal syndrome presents as abnormal LFTs, splenomegaly, ascites, and thrombocytopenia. It is due to increased flow in the splenic artery away from the hepatic graft, causing graft hypoperfusion. Steal syndrome has also been reported with the gastroduodenal artery. Imaging findings:
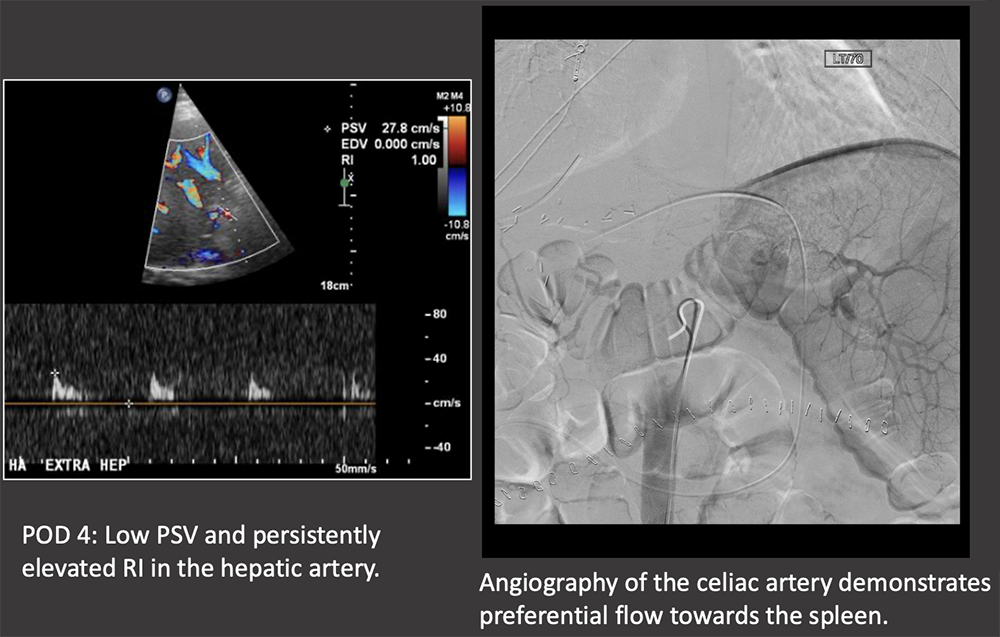 |
Case: Splenic Steal Syndrome Low PSV in the hepatic artery (<35 cm/s) should prompt consideration of splenic steal syndrome. Definitive diagnosis is with conventional angiography. Treatment is splenic artery embolization. Li, 2017; Horrow, 2020 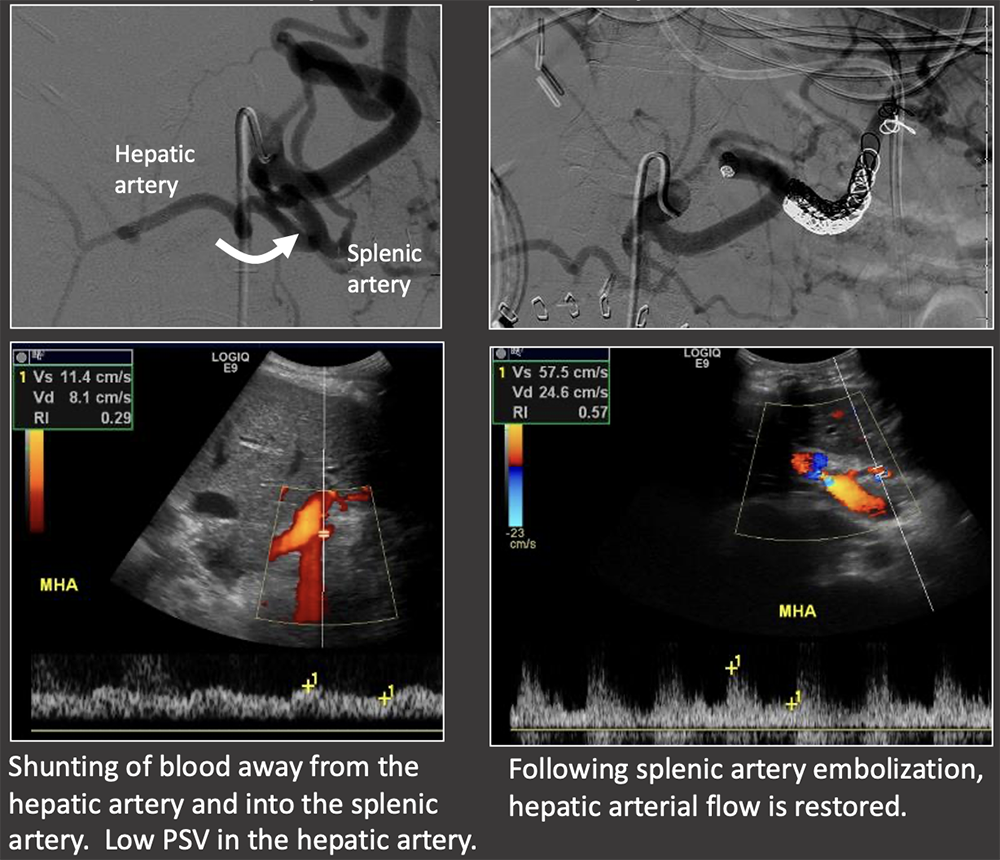 |
Case: Hepatic Artery Pseudoaneurysm Hepatic artery pseudoaneurysms are rare complications. Extrahepatic pseudoaneurysms are more common and tend to occur at the site of anastomosis or angioplasty, and are often mycotic. Intrahepatic pseudoaneurysms are less likely and tend to occur at the site of graft biopsy or focal infection. Vascular intervention is required to avoid complications such as rupture, or fistulization with the biliary or gastrointestinal tracts. Garcia-Criado, 2009; Kimura, 2020; Vaidya, 2007 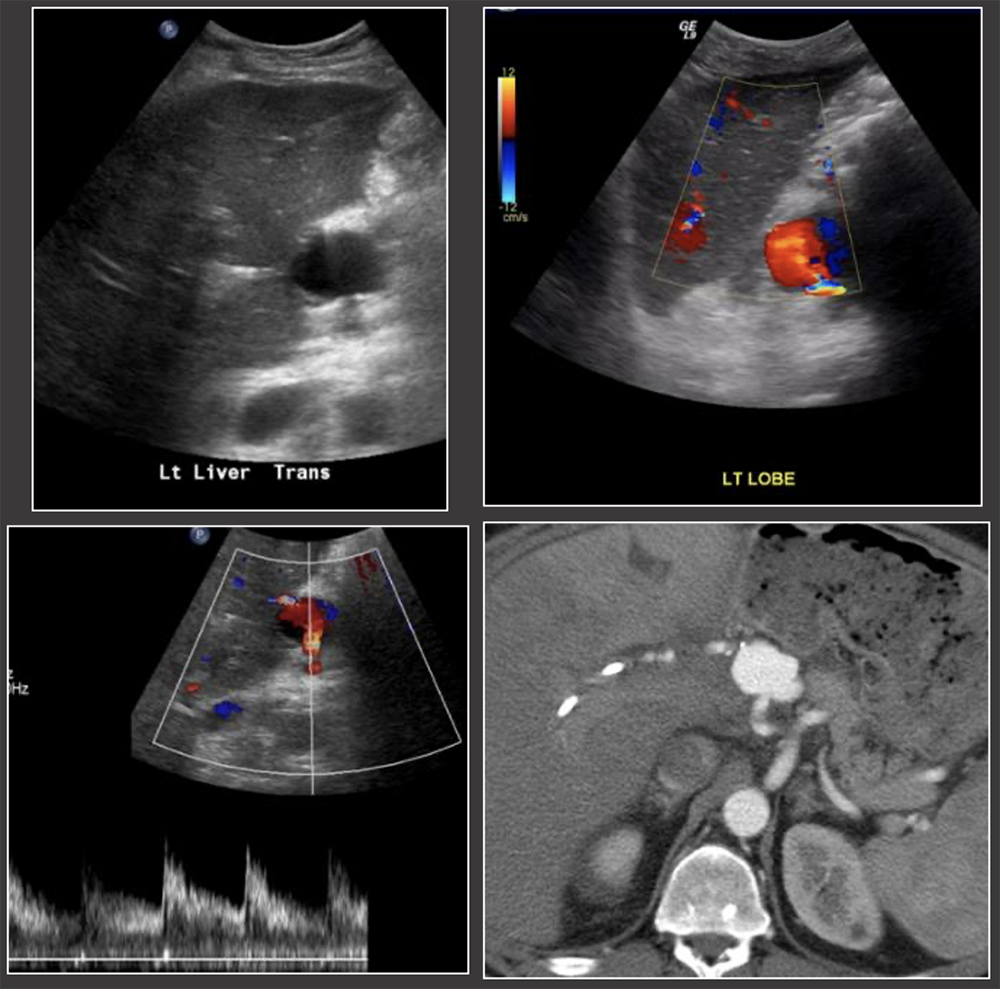 |
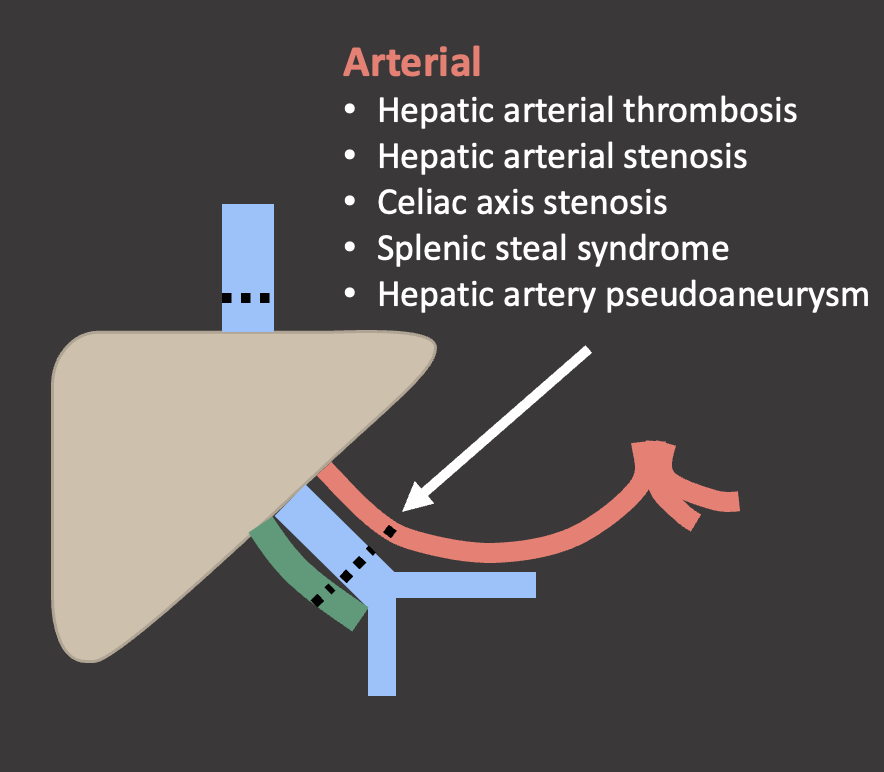
Hepatic Arterial Complications Summary 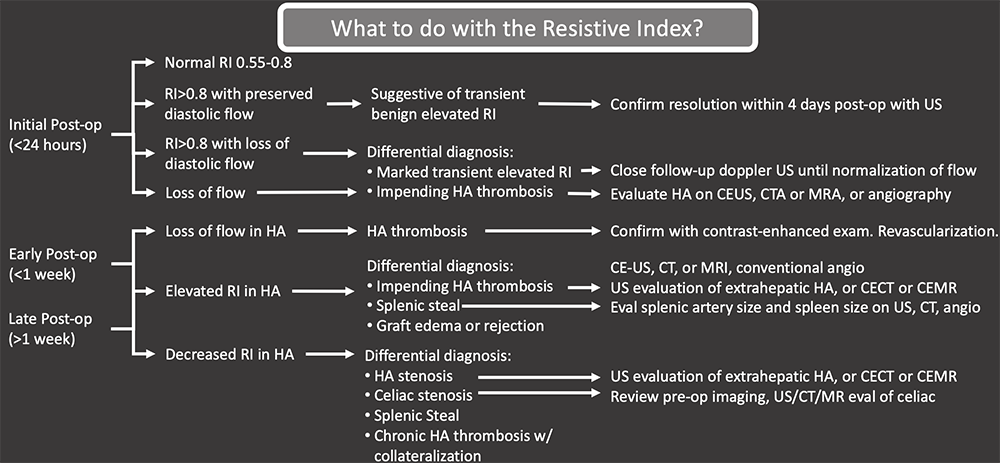 |
Portal Vein Normal Appearance in OLT Portal vein anastomosis in OLT
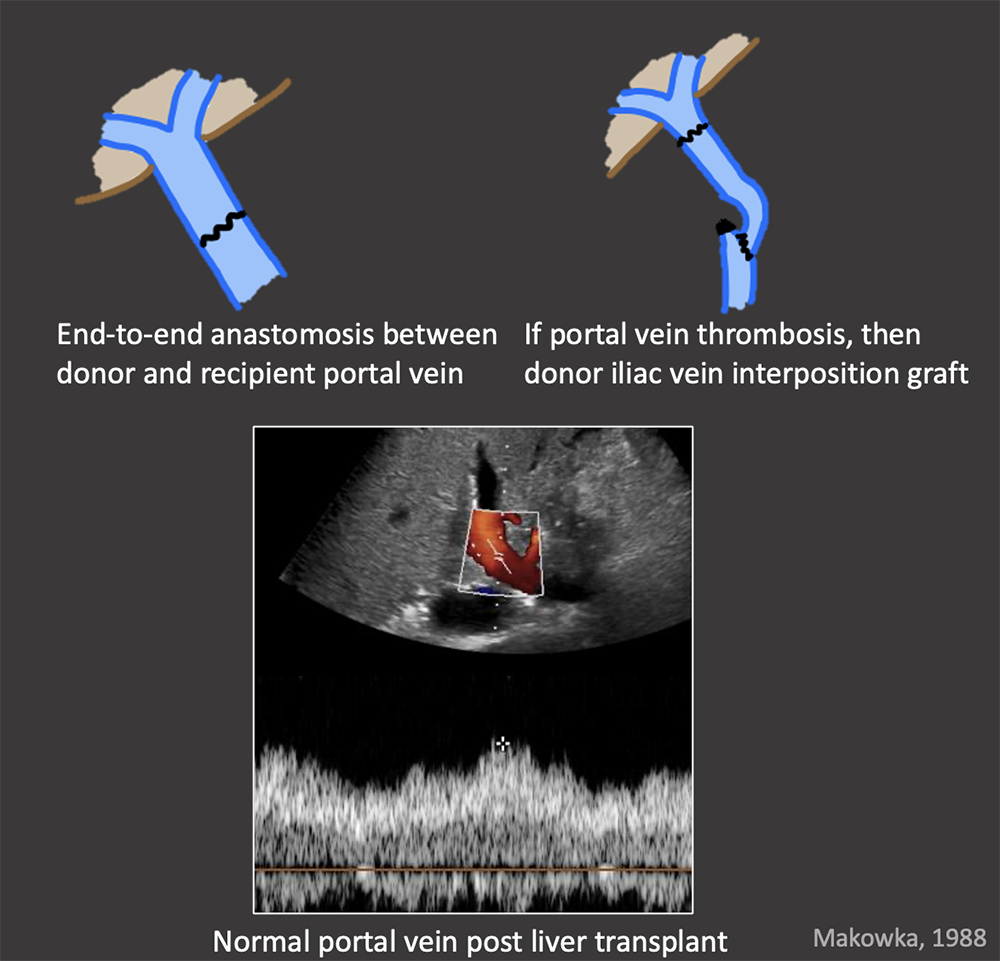 |
Case: Portal Vein Thrombosis Portal venous complications are less common the arterial complications. When they do occur, they typically occur at the anastomosis. Risk factors for portal venous thrombosis:
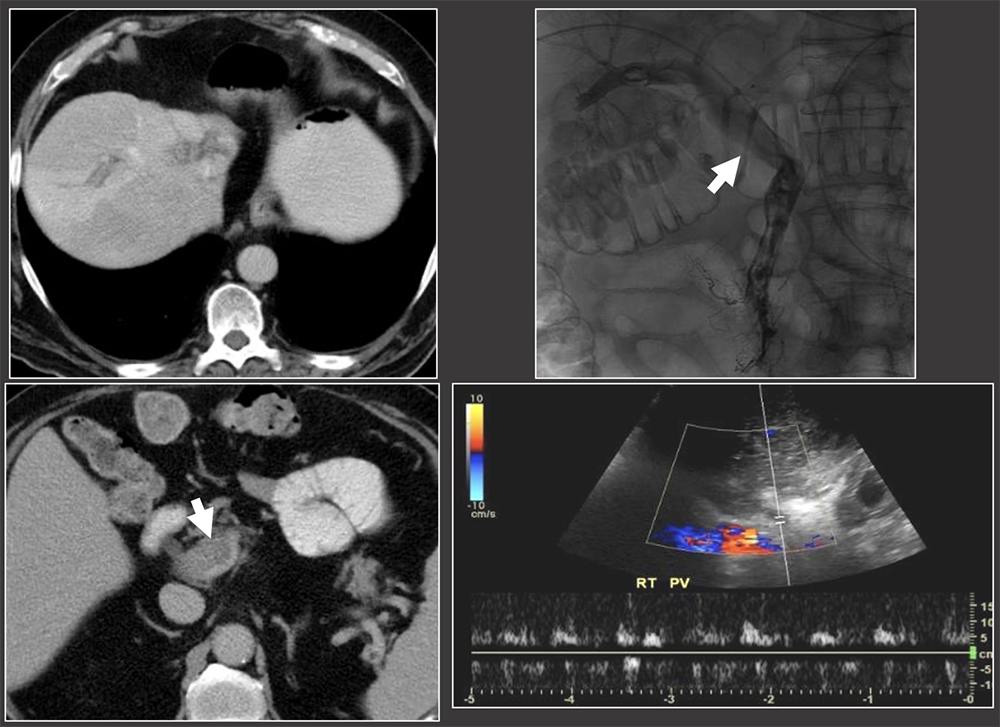 |
Case: Portal Vein Stenosis Imaging findings of portal venous stenosis:
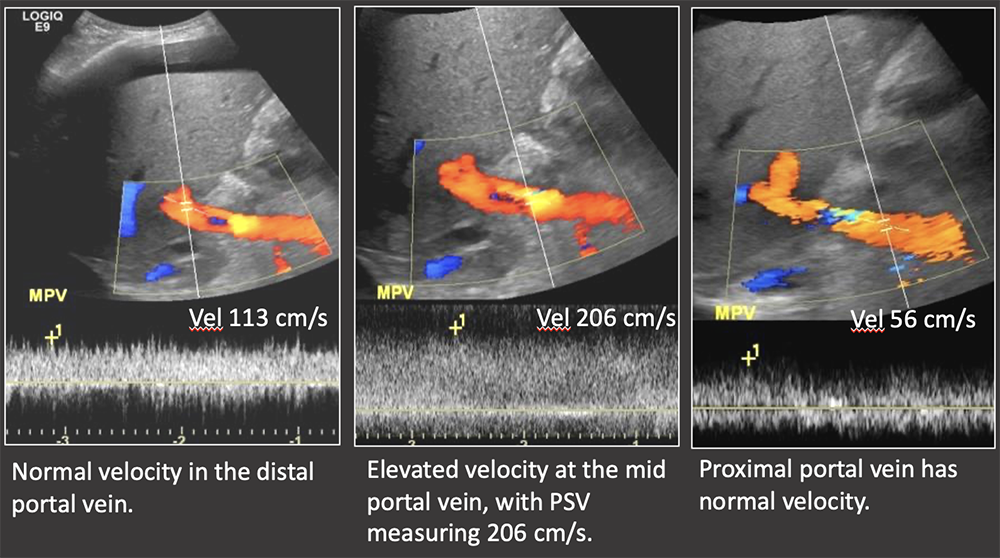 |
Hepatic Vein & IVC Normal Appearance in OLT Multiple surgical techniques exist for the IVC anastomosis.
Bhargava,2011; Ahlawasi, 2012; Crossin, 2003 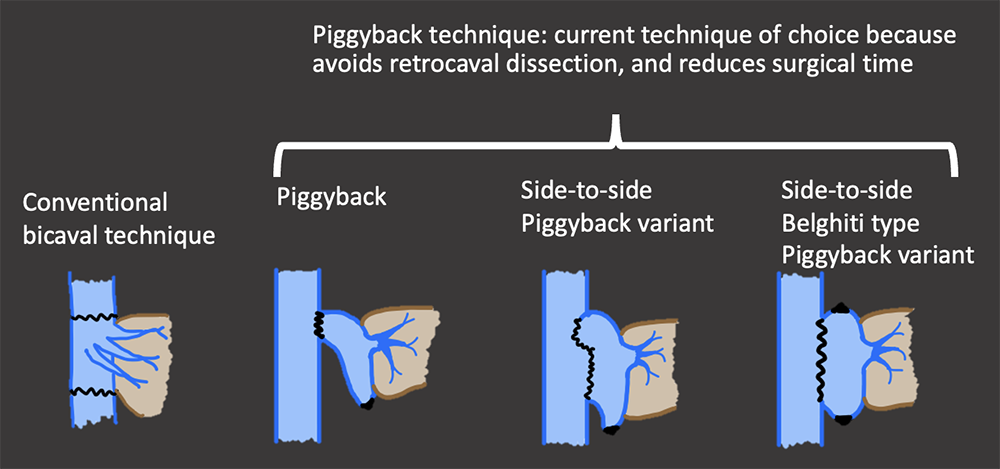 |
Hepatic Vein & IVC Normal Appearance in OLT Normal US evaluation of the middle hepatic vein. Patient status post deceased donor transplant with side-to-side cavocavostomy. 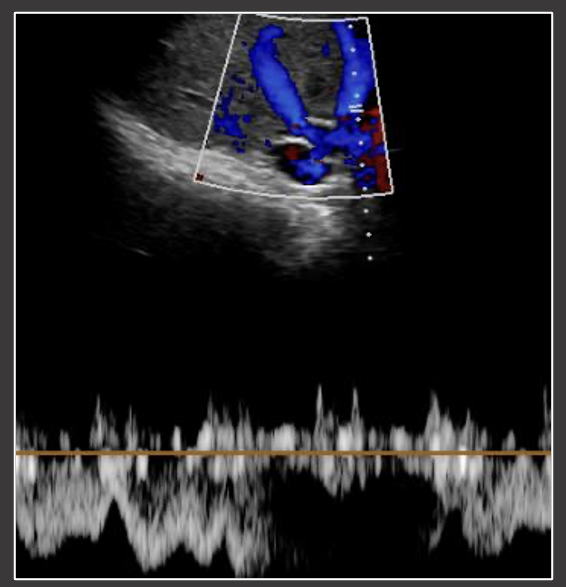 |
Case: Hepatic Vein and IVC Thrombosis IVC and hepatic venous complications are rare in OLT and can cause graft dysfunction or failure. Post-transplant ultrasound should routinely evaluate both the hepatic veins as well as the piggyback anastomosis. Kimura, 2020 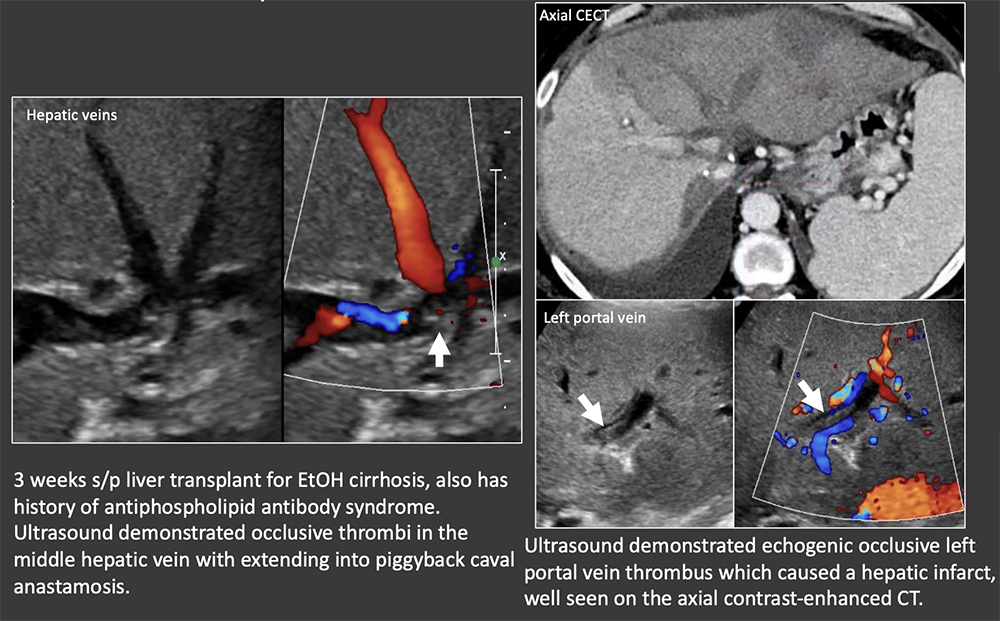 |
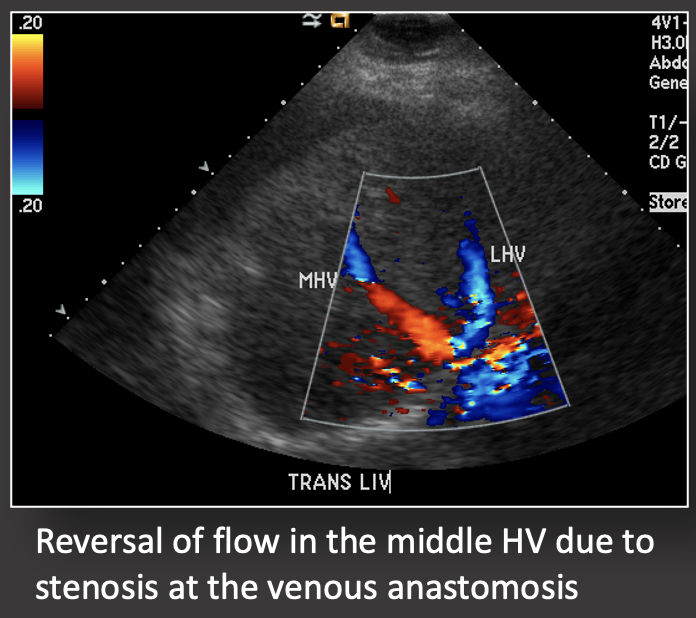
Case: IVC Stenosis Hepatic vein and IVC complications are more common in living donor transplants because of the more complex venous reconstruction. Risk factors for hepatic vein/IVC stenosis:
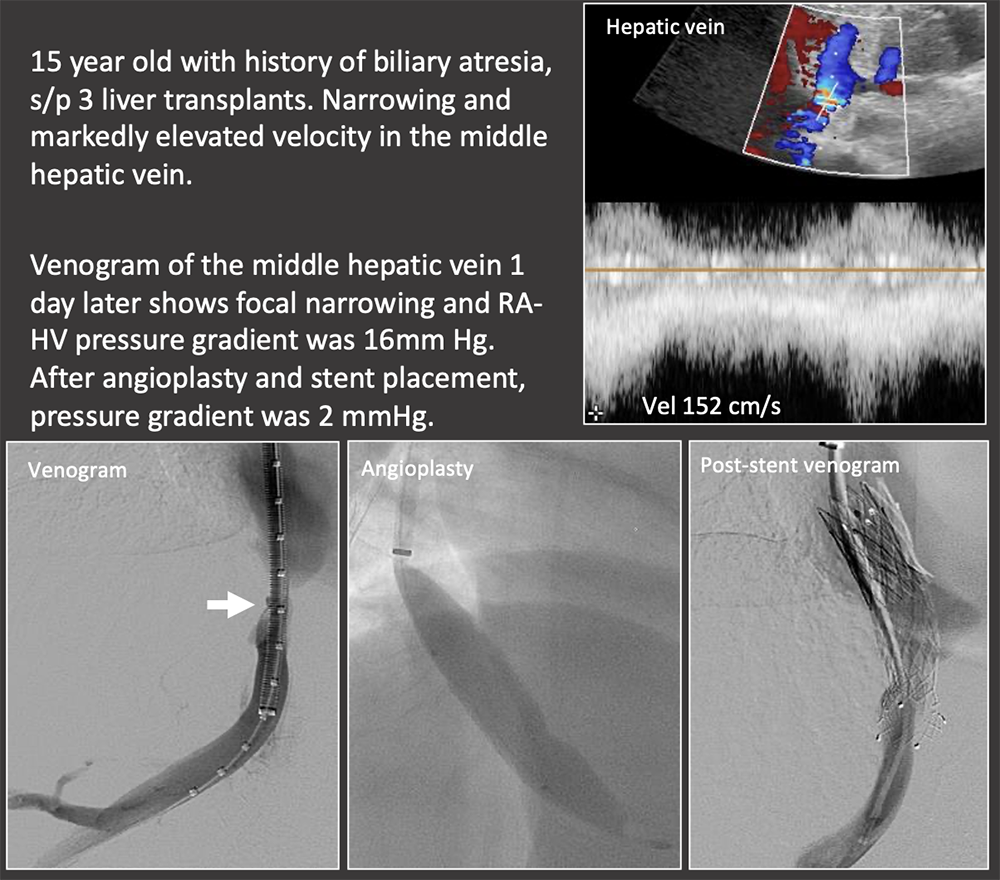 |
Case: Graft Torsion Graft torsion may be a cause of hepatic venous outflow obstruction in living donor liver transplants. HV outflow obstruction is graft threatening because it causes significant parenchymal congestion which compromises arterial and portal inflow, as seen in this case with portal flow reversal. Differential diagnosis for hepatic venous outflow obstruction in living donor liver transplant:
Treatment is surgical graft repositioning and hepatopexy. Abdelaziz, 2016; Jeng, 2017 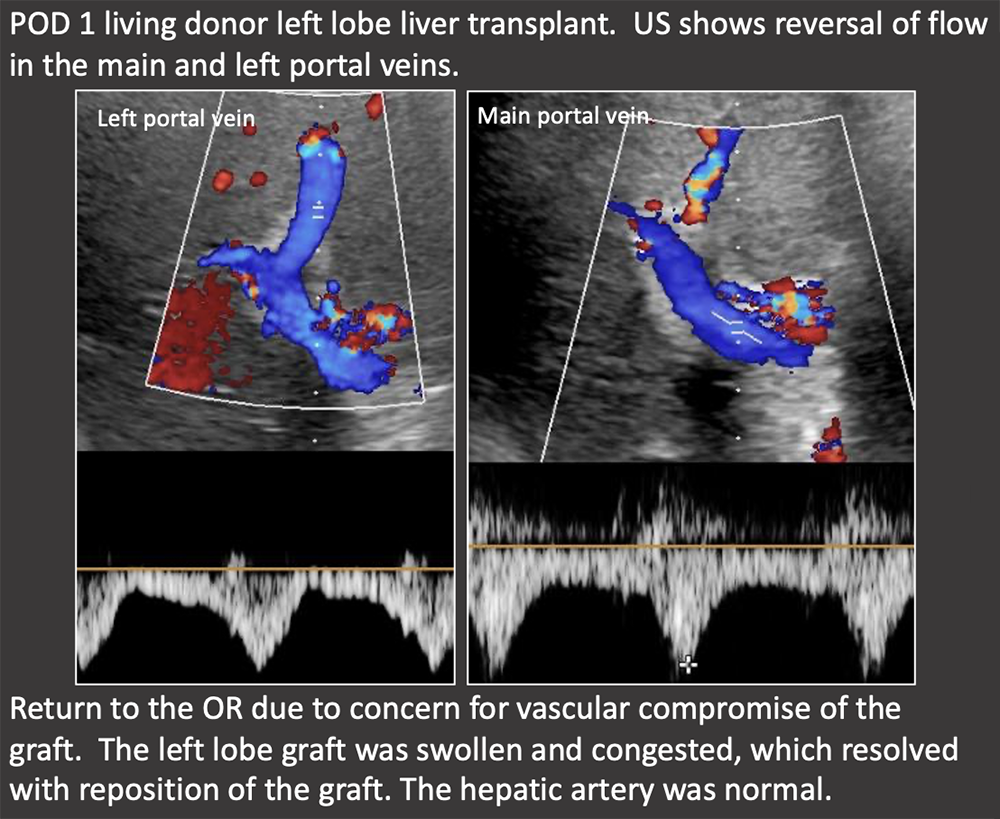 |
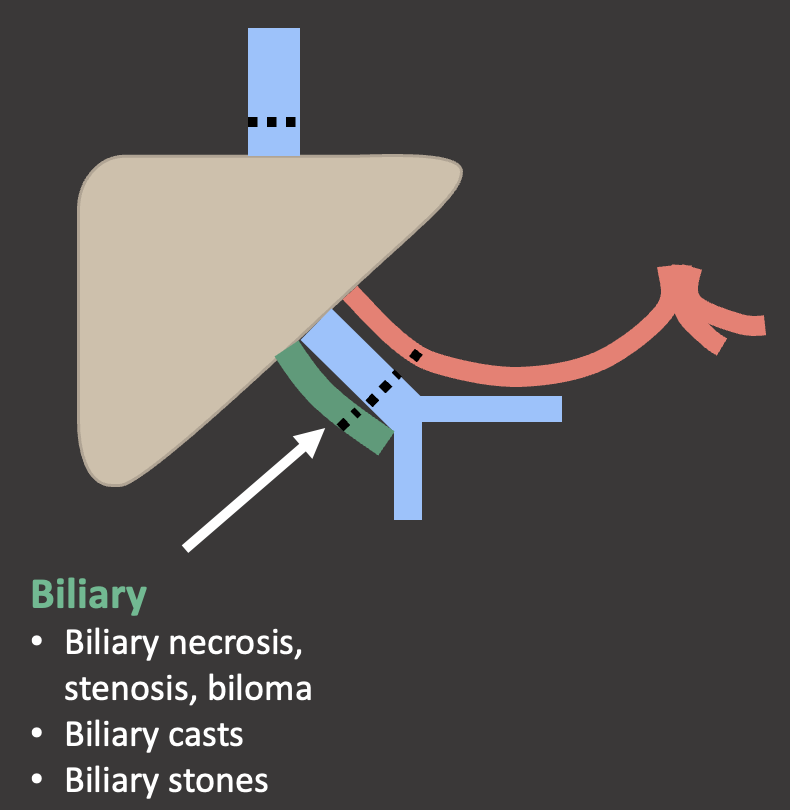
Biliary Anastomosis in OLT Biliary anastomosis
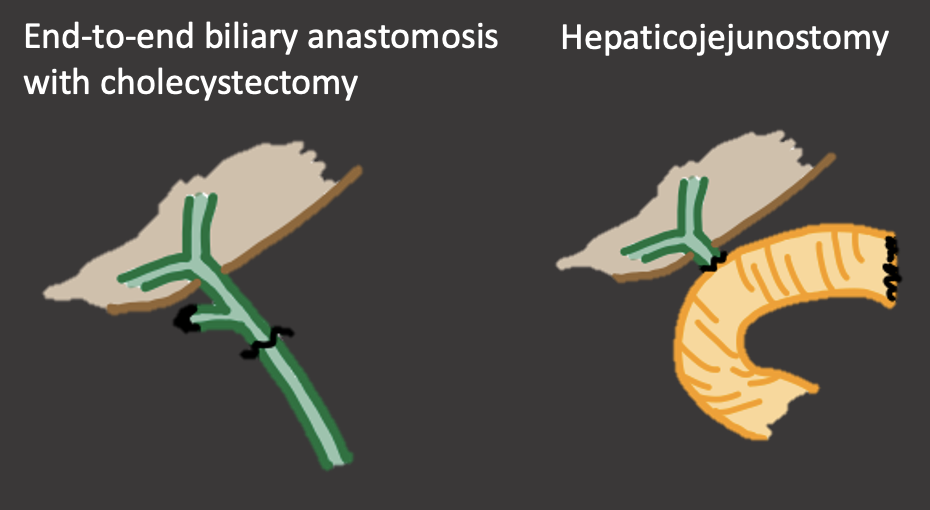 |
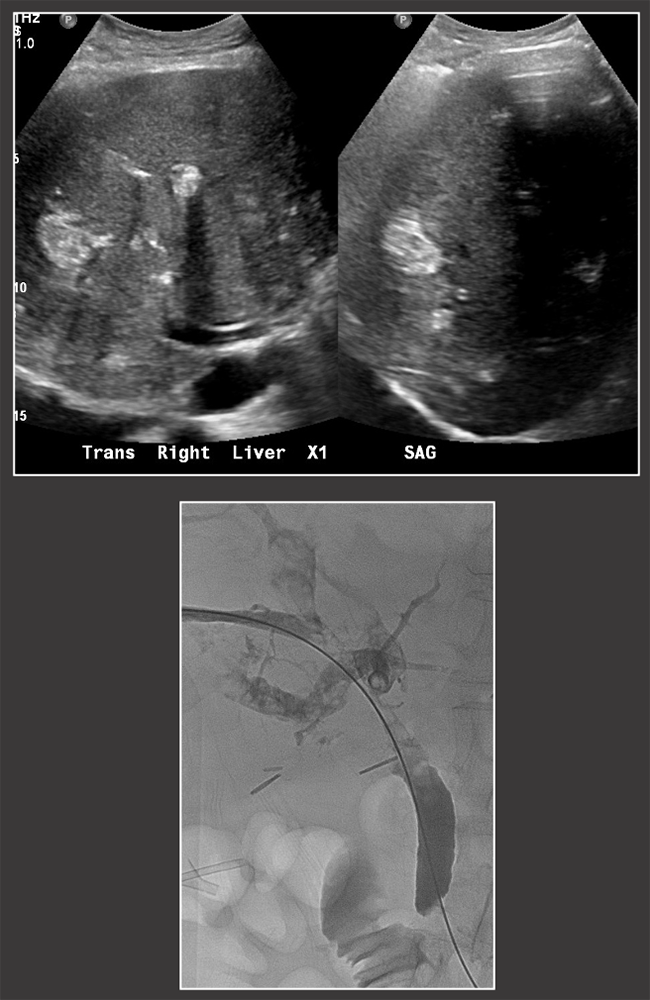
Case: Biliary Stones Biliary stones tend to occur >1 year post-transplant, although are overall much less common than biliary strictures or leaks. Biliary stones or sludge suggest underlying biliary stasis, which may be due to stricture. Biliary stones may also be medication-induced, such as with cyclosporine. Biliary stones can cause obstruction and cholangitis and often require percutaneous, endoscopic, or surgical removal. Crossin, 2003 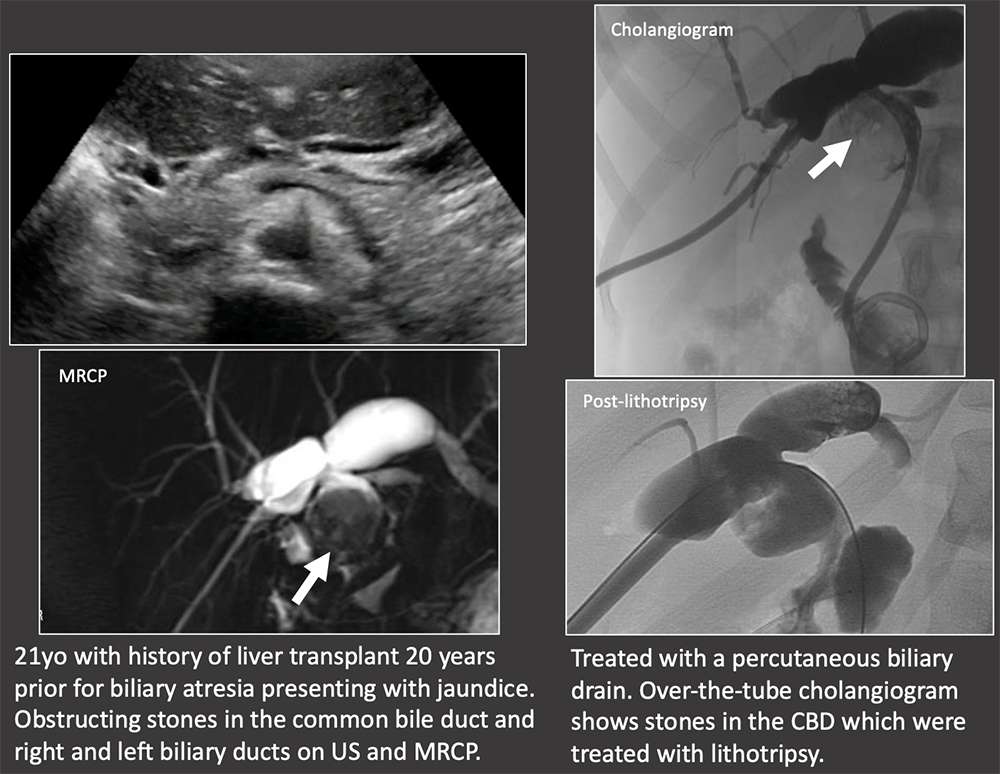 |
Case: Biliary Cast Syndrome Biliary cast syndrome is due to biliary ischemia with sloughing of the biliary mucosa. It is usually due to underlying hepatic artery thrombosis or stenosis, therefore biliary casts on exam should prompt close evaluation of the hepatic artery. Hu, 2016 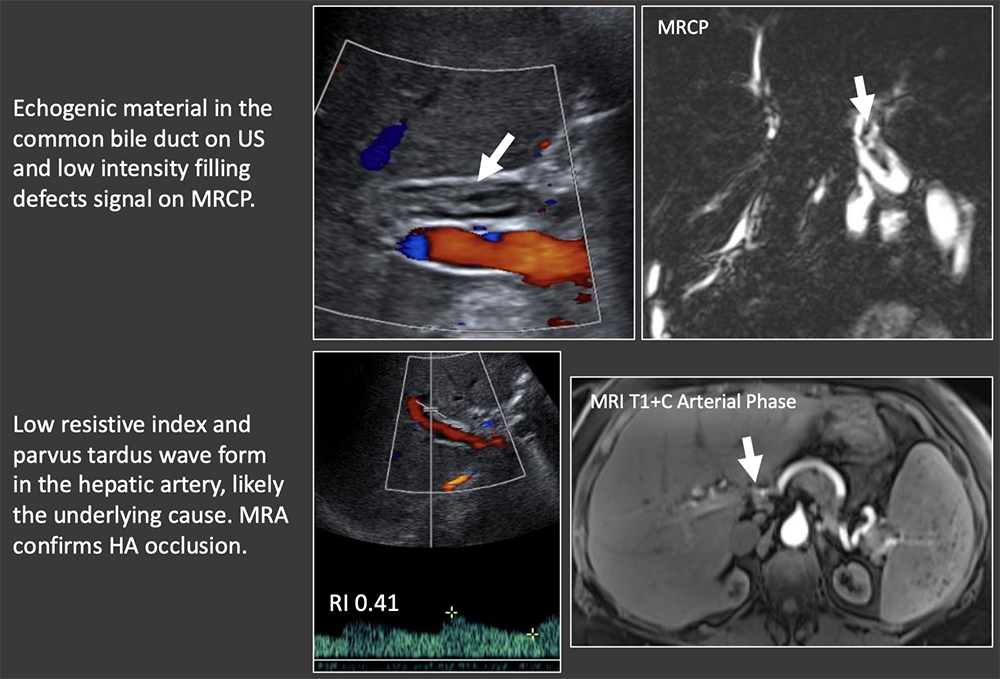 |
Case: Biliary Cast Syndrome Imaging findings:
Hu, 2016 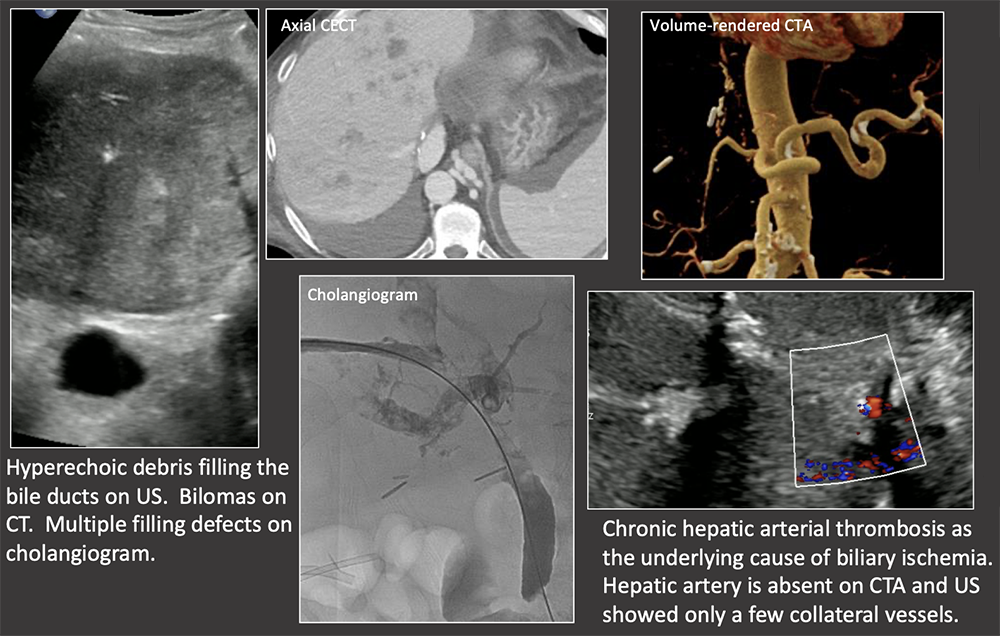 |
Summary of Case Teaching Points
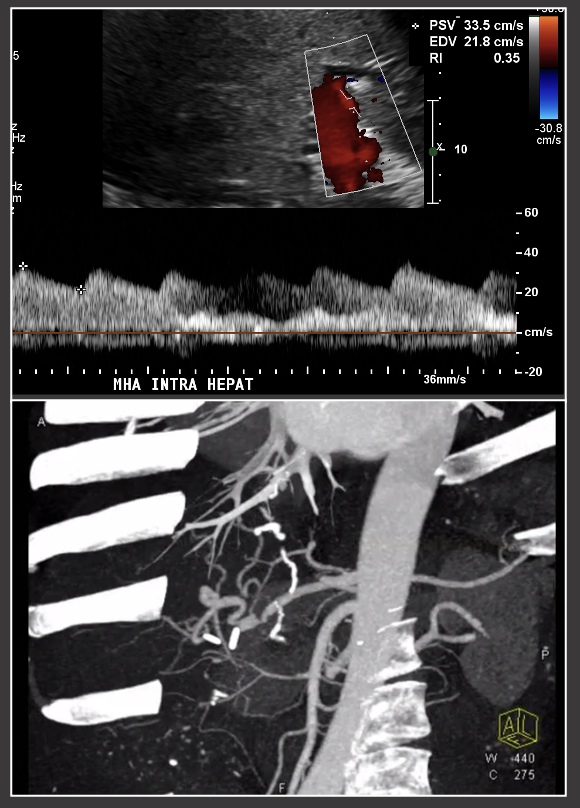  |
Summary of Case Teaching Points Portal venous complications are uncommon. 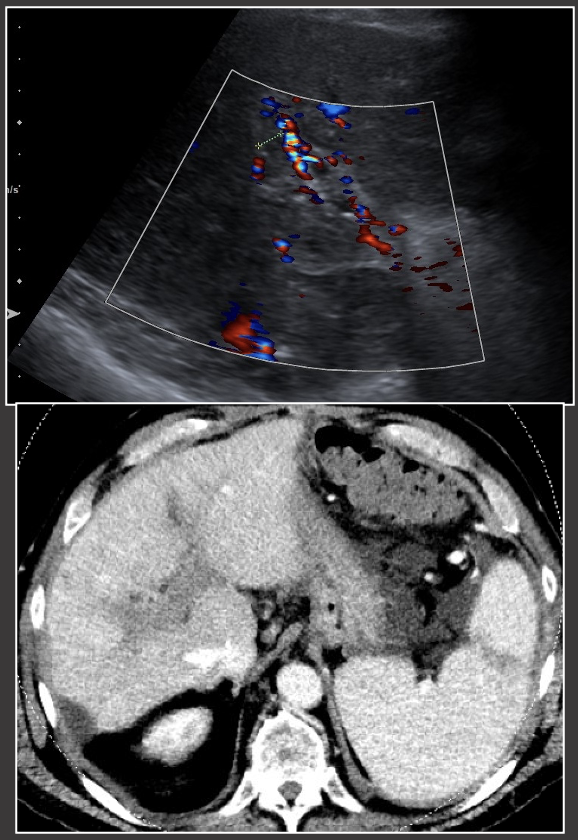 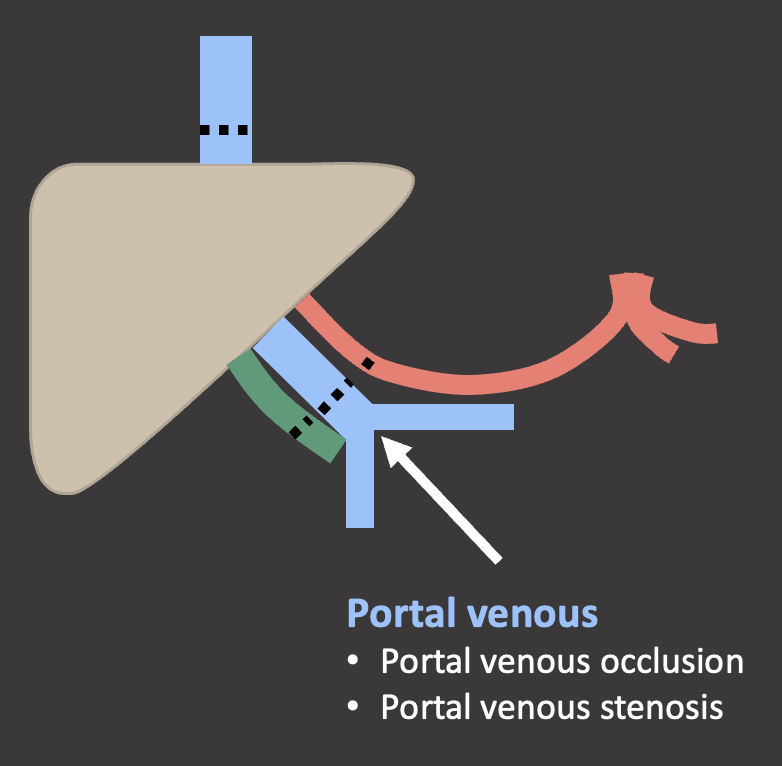 |
Summary of Case Teaching Points
 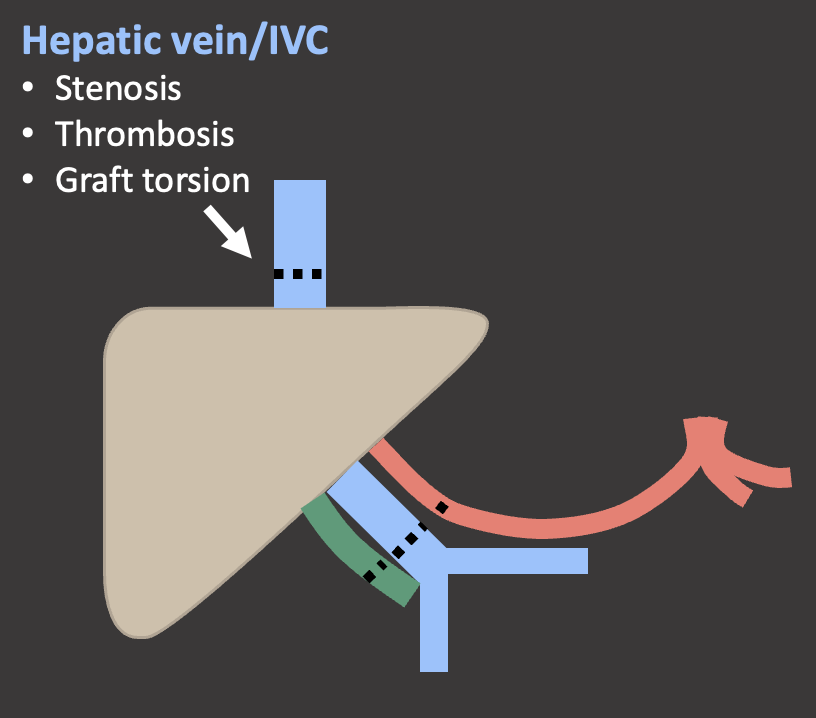 |
Summary of Case Teaching Points
  |
Summary of Case Teaching Points
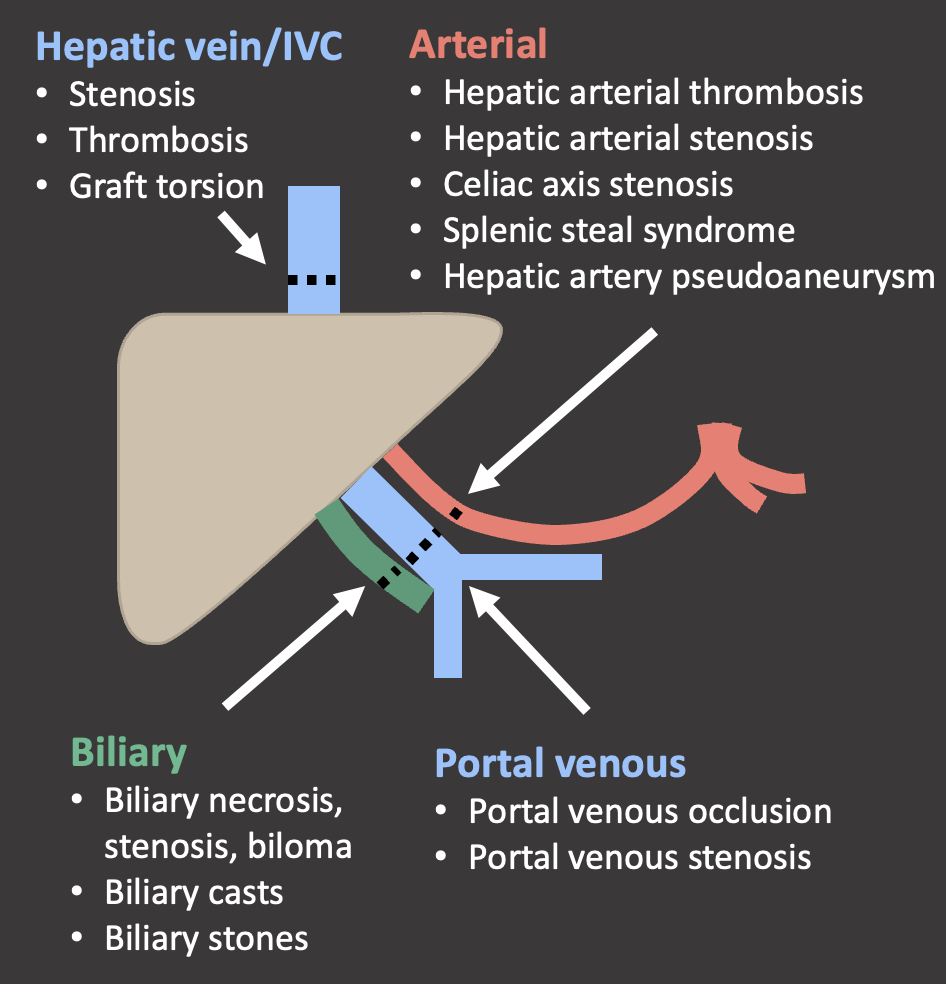 |
References
|
