Typical and Atypical Appearances of Pancreatic Neuroendocrine Tumors (PNETs): Role of CT Angiography (CTA) and Cinematic Rendering (CR)
Typical and Atypical Appearances of Pancreatic Neuroendocrine Tumors (PNETs): Role of CT Angiography (CTA) and Cinematic Rendering (CR) |
What is PNET (aka Pancreatic Neuroendocrine Tumor)?
 |
Variable Appearances SHAPE/SIZE: typically well-circumscribed, ranging from small to large SOLID/CYSTIC: typically solid however can also be cystic and/or necrotic ENHANCEMENT: typically hypervascular, however can be hypovascular VASCULAR INVOLVEMENT: typically invade venous vasculature rather than encasing and narrowing them CALCIFICATIONS: can occur, typically central/bulky METASTASES: typically hypervascular lesions - most commonly to the liver and lymph nodes; lesions/nodes can be hypovascular after treatment DUCTAL INVOLVEMENT: rare, but can occur if tumor is extensively large or PNET that secretes serotonin which leads to ductal fibrosis and obstruction |
Differential Diagnosis PNET
|
Pancreas Protocol Importance of protocol optimization
|
Dual Phase Imaging for Pancreas
|
Cinematic Rendering
|
Staging/Management AJCC 8th Edition Staging
|
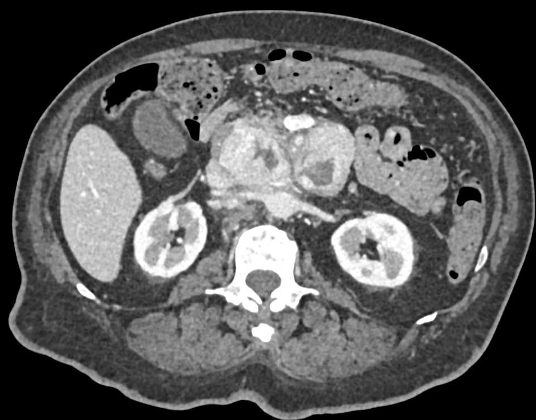
Case 1: Typical Hypervascular PNET Teaching point(s):
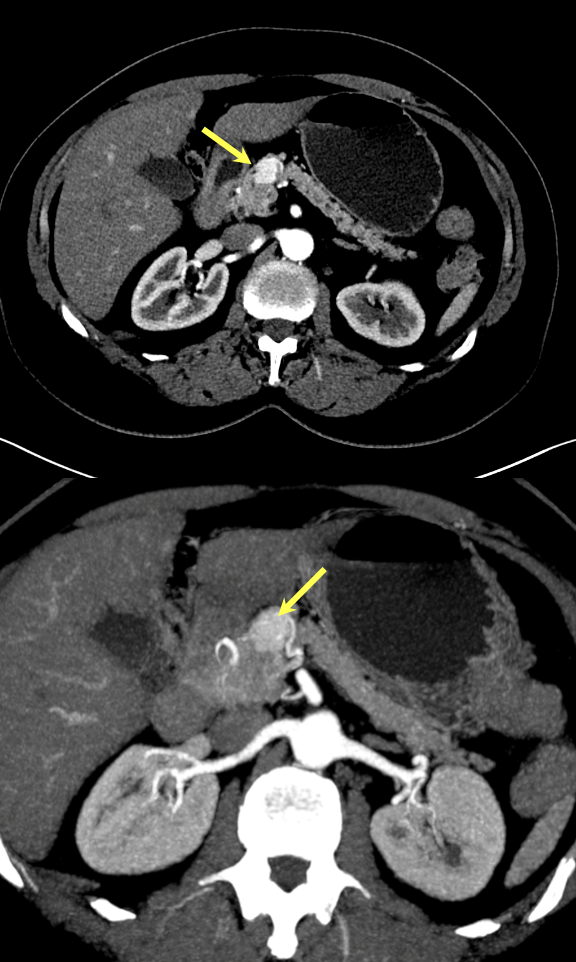 |
Case 1: Typical Hypervascular PNET (cont’d) - cinematic renderings 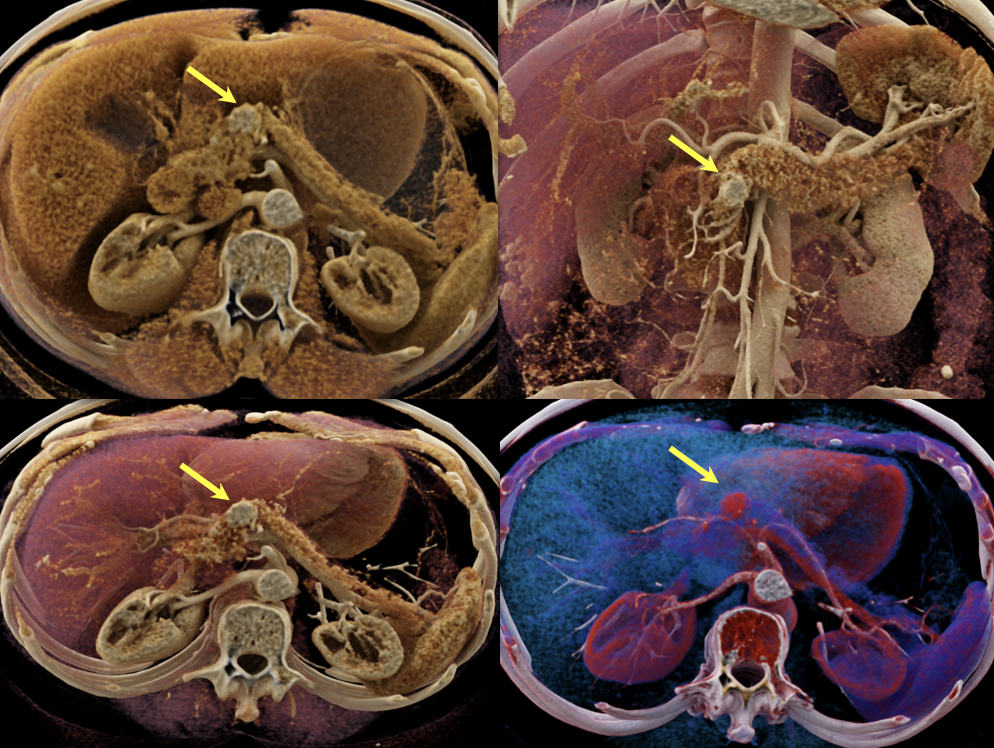 |
Case 2: Well-differentiated PNET Teaching point(s):
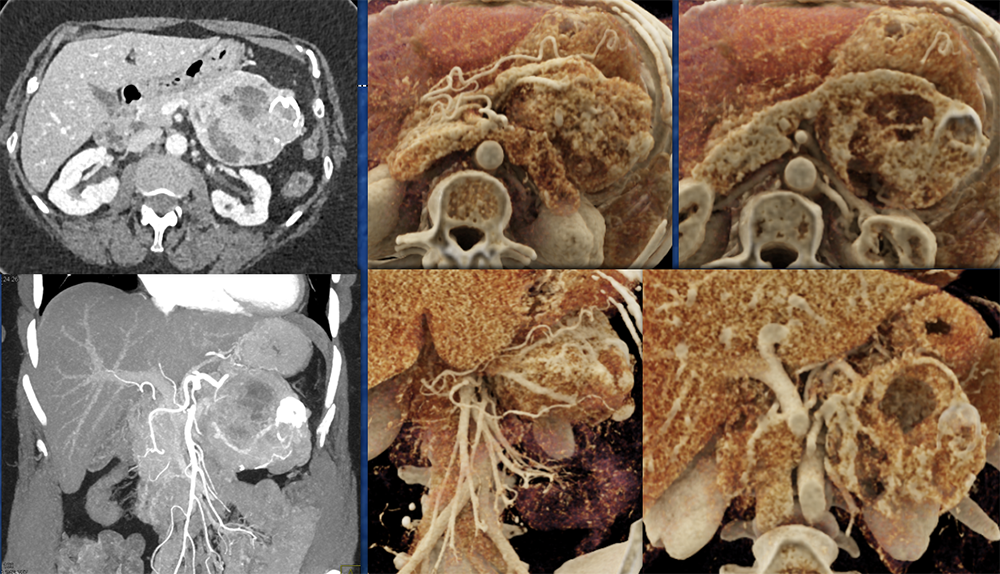 |
Case 3: Atypical Hypovascular PNET Teaching point(s):
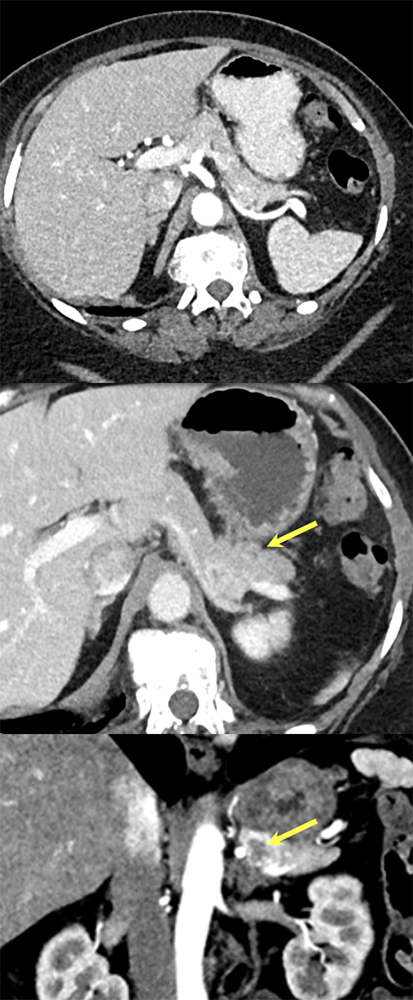 |
Case 3: Atypical Hypovascular PNET (cont’d) 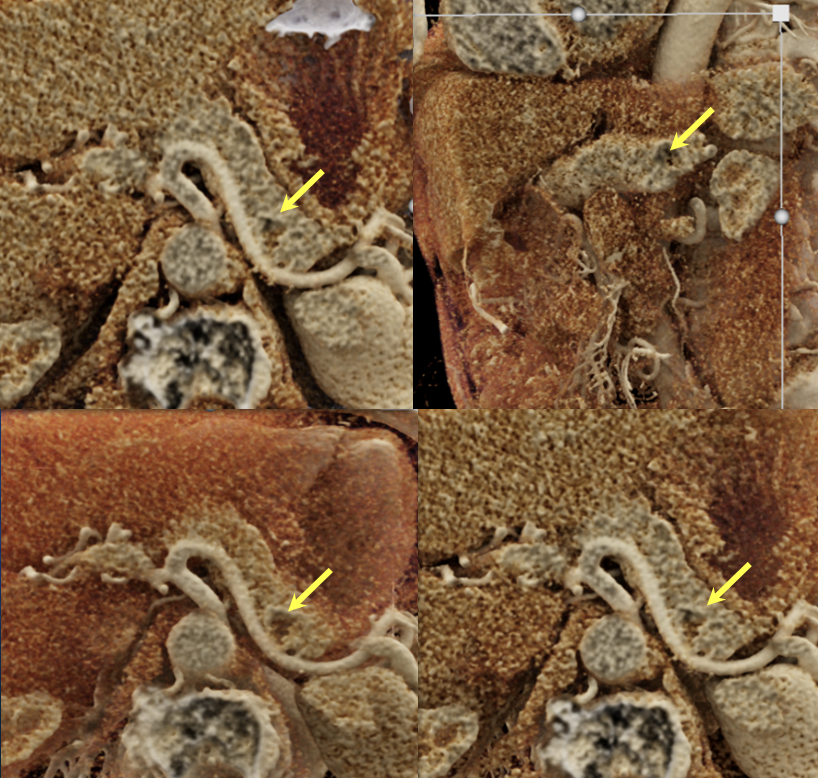 |
Case 4: Incidentally noted PNET Teaching point(s):
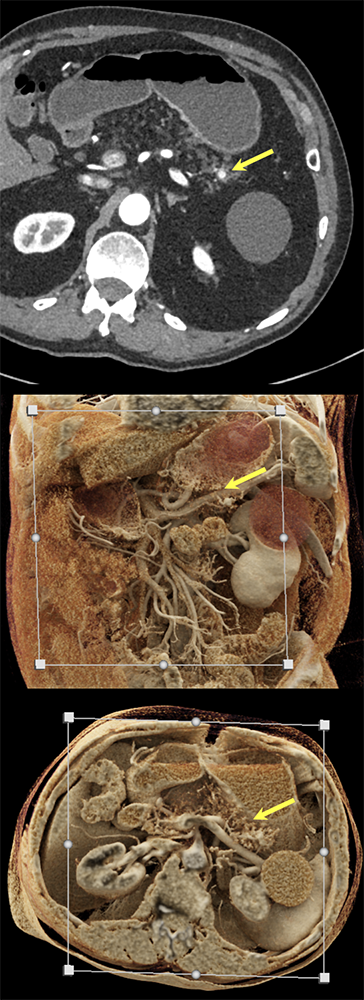 |
Case 5: Incidentally noted PNET Teaching point(s):
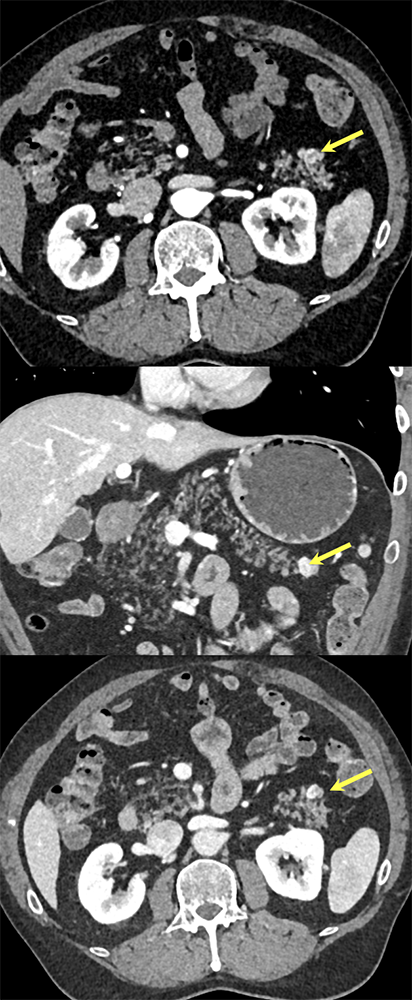 |
Case 5: Incidentally noted PNET (cont’d)  |
Case 6: Multiple PNETs in patient with Von Hippel Lindau Teaching point(s):
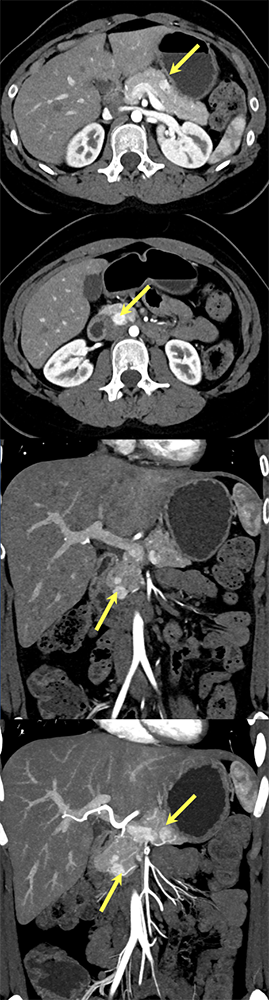 |
Case 6: Multiple PNETs in patient with Von Hippel Lindau (cont’d) 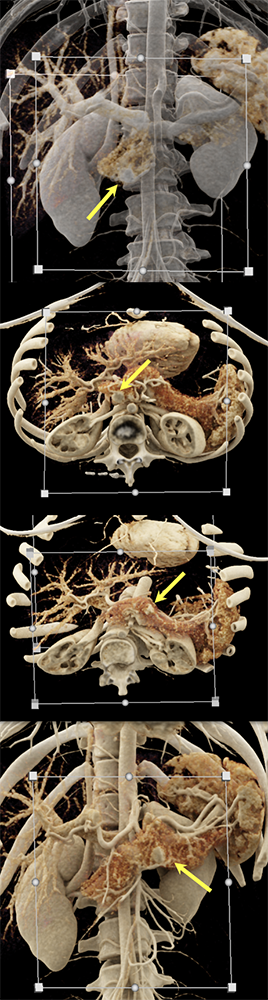 |
Case 7: PNET with Hepatic Metastases and Splenic Vein Occlusion Teaching point(s):
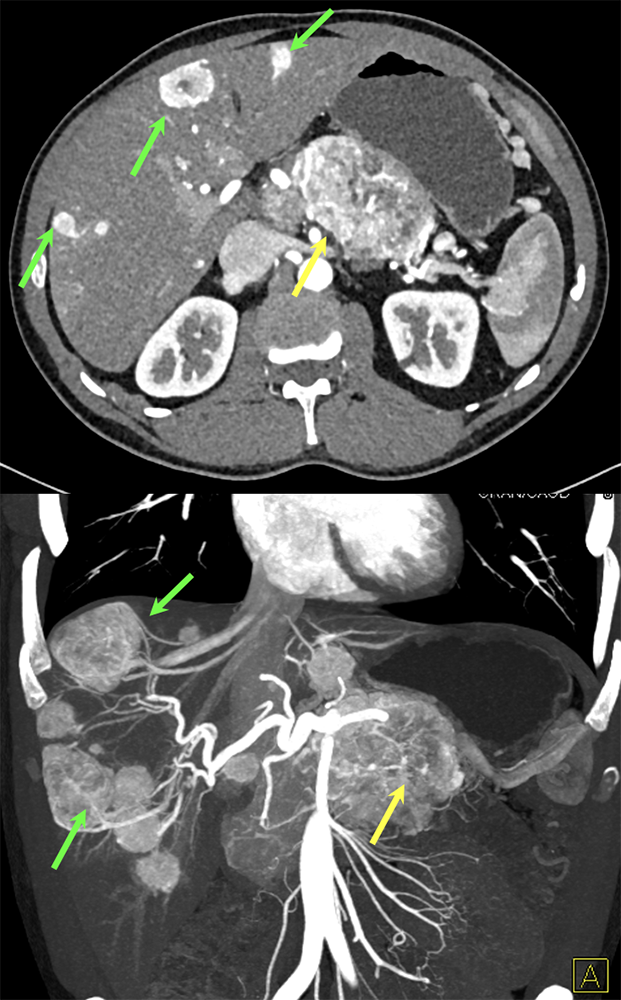 |
Case 7: PNET with Hepatic Metastases and Splenic Vein Occlusion Teaching point(s):
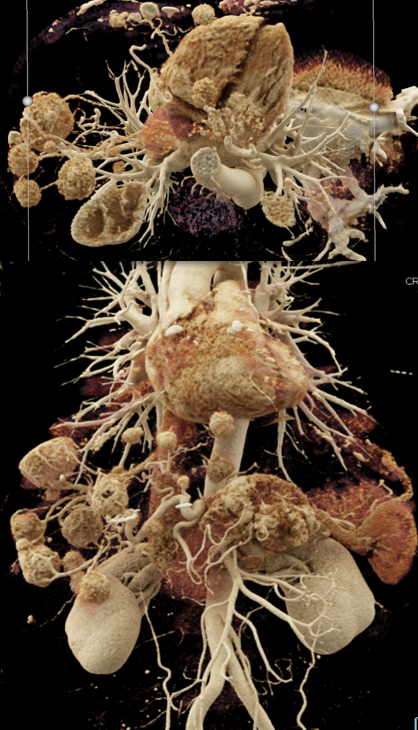 |
Case 8: PNET with Metastases and Vascular Involvement Teaching point(s):
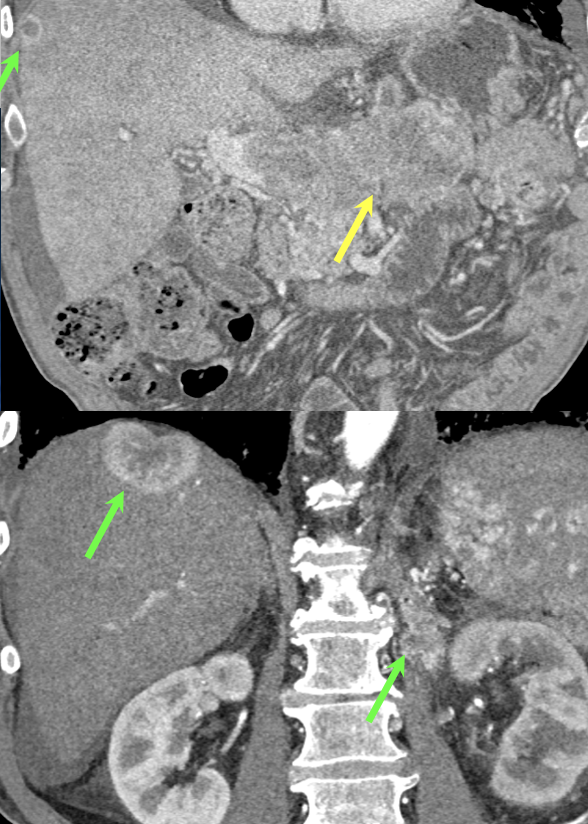 |
Case 8: PNET with Metastases and Vascular Involvement (cont’d) 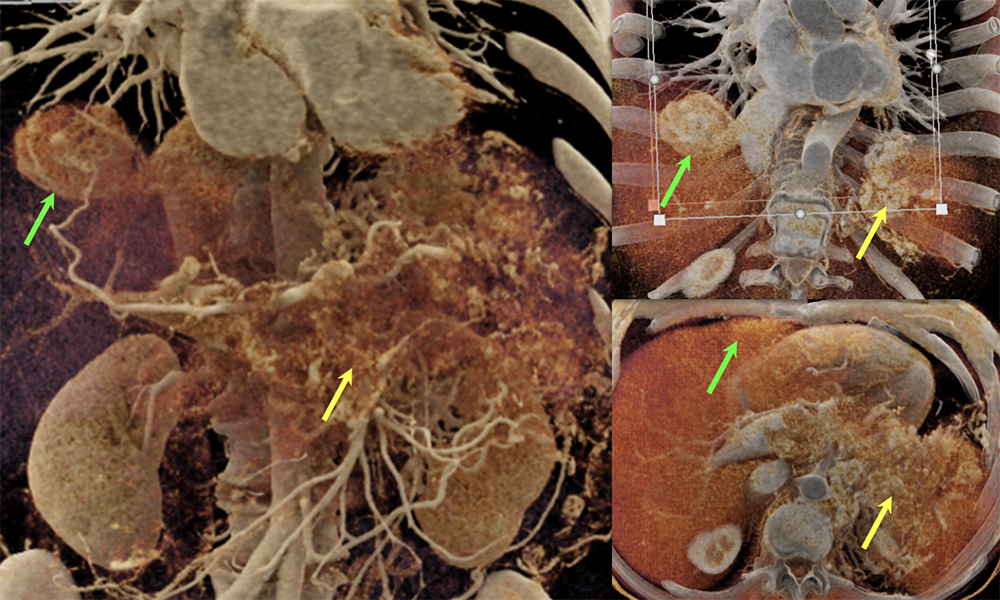 |
Case 8: PNET with Metastases and Vascular Involvement (cont’d) Teaching point(s):
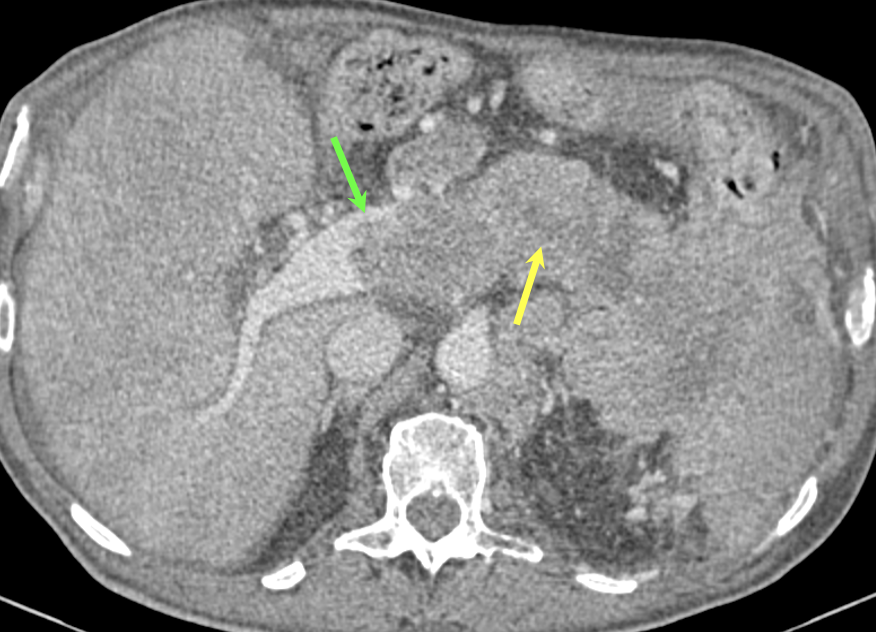 |
Case 9: PNET with calcification Teaching point(s):
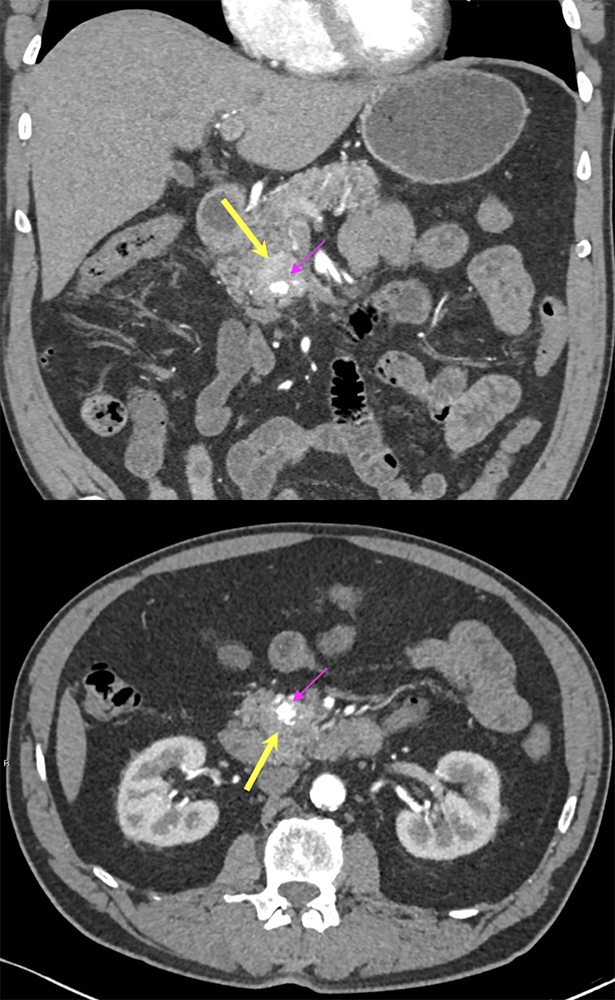 |
Case 9: PNET with calcification (cont’d) 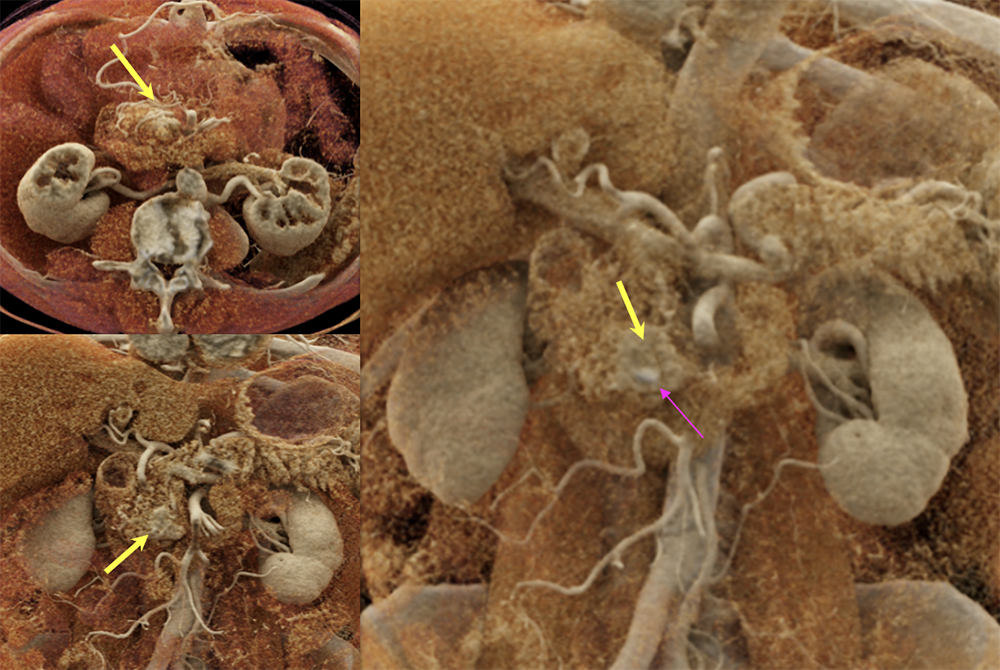 |
Case 10: Cystic PNET (uncinate) Teaching point(s):
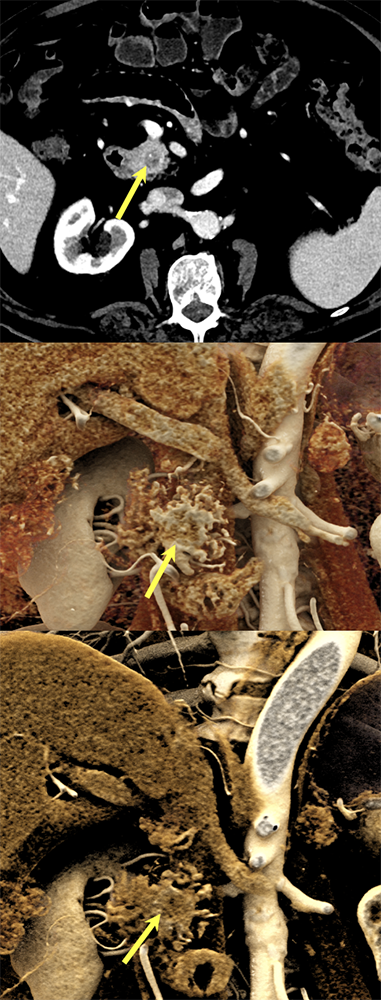 |
Case 11: Cystic PNET (Tail) 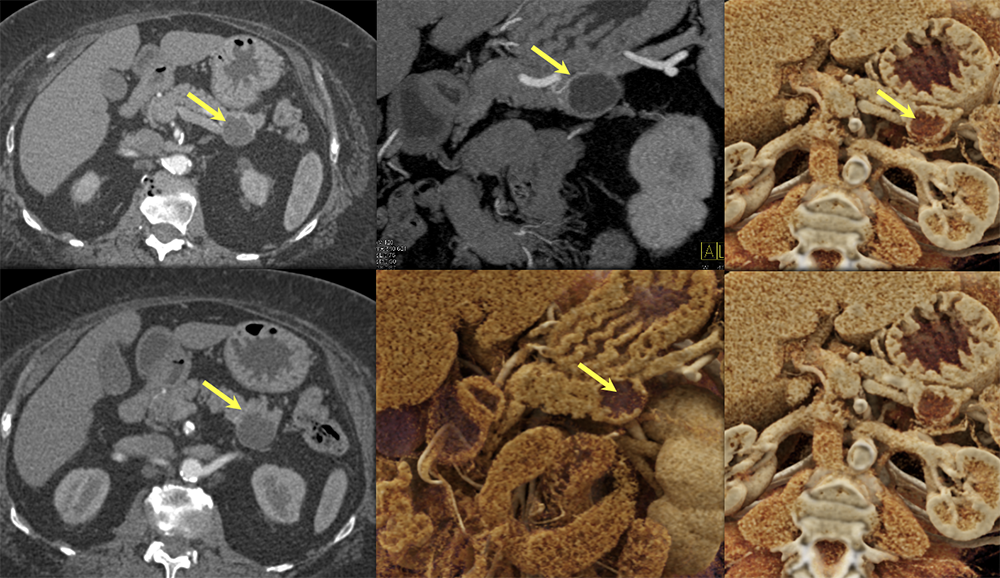 |
Case 12: PNET with Central Necrosis 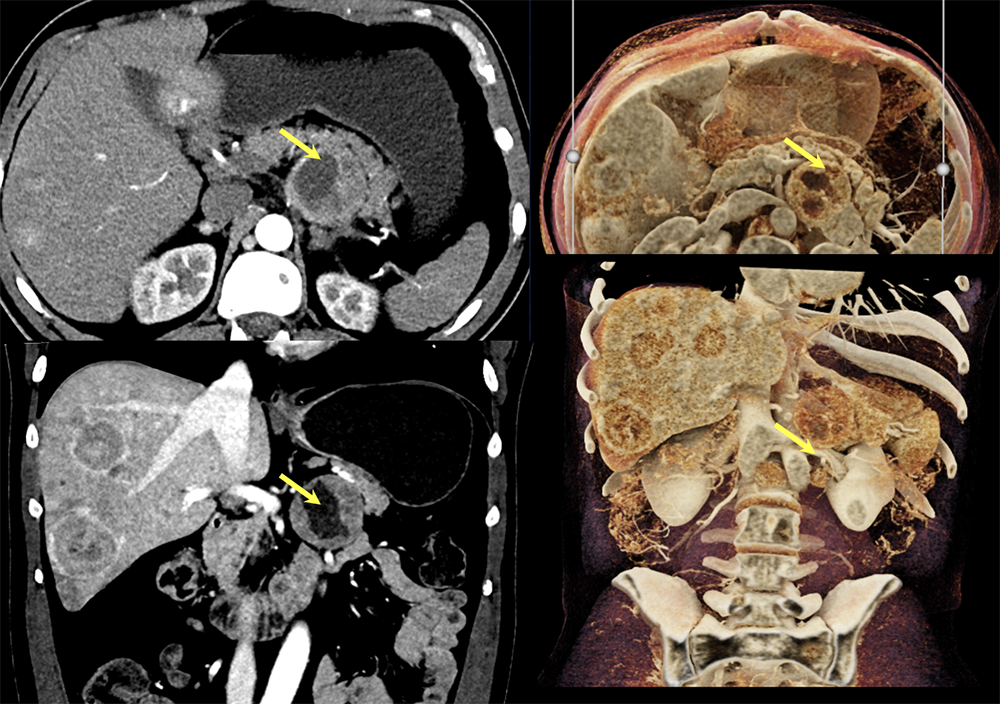 |
Case 13: Pancreatic Tail PNET, 1cm 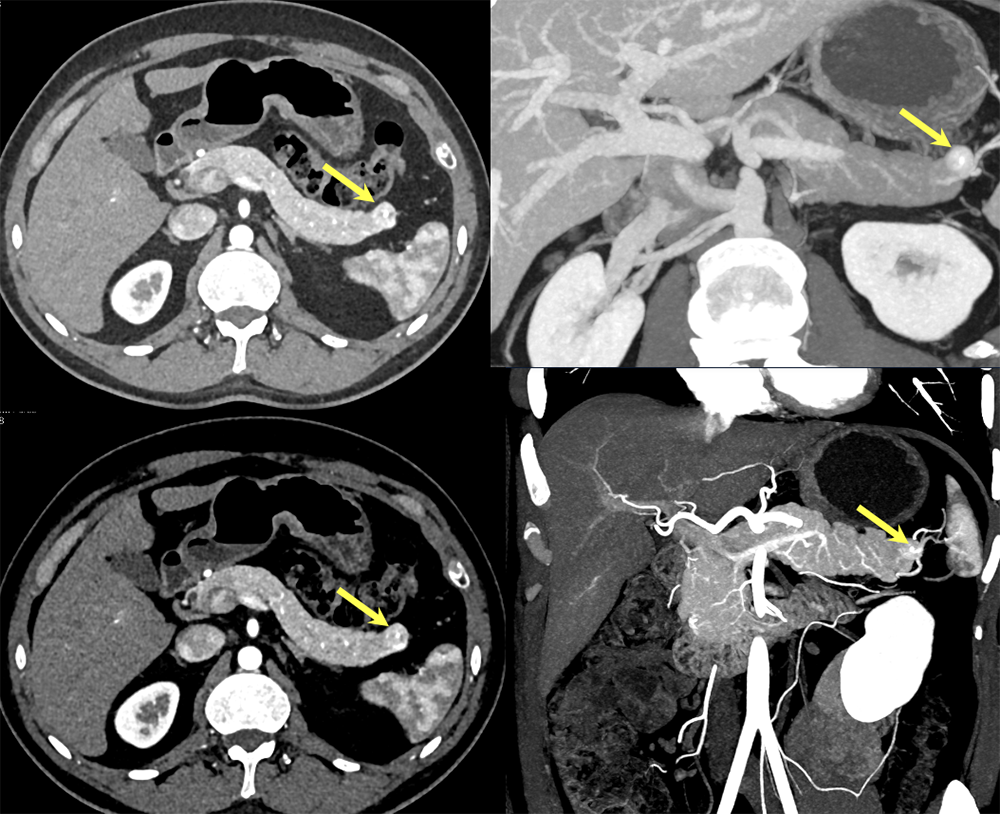 |
Case 13: Pancreatic Tail PNET, 1cm Teaching point(s):
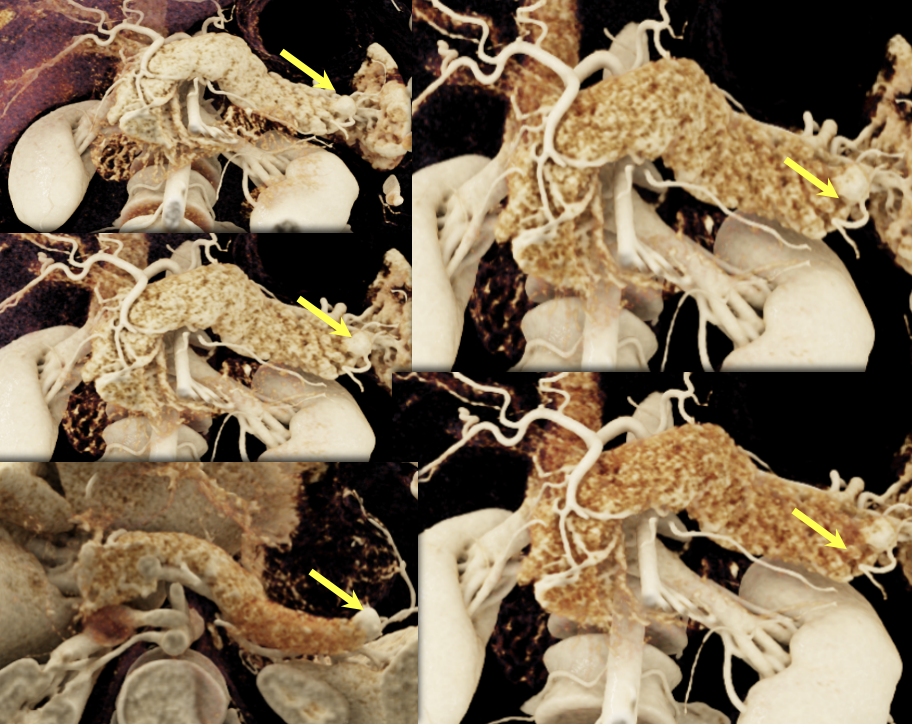 |
Case 14: PNET Obstructing the Pancreatic Duct 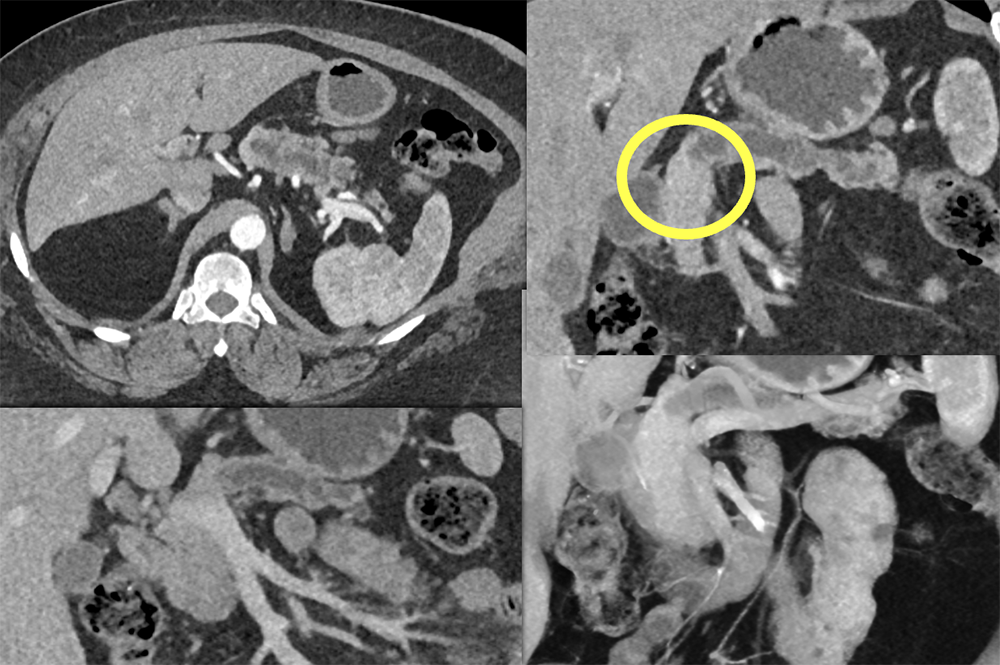 |
Case 14: PNET Obstructing the Pancreatic Duct (cont’d) Teaching point(s):
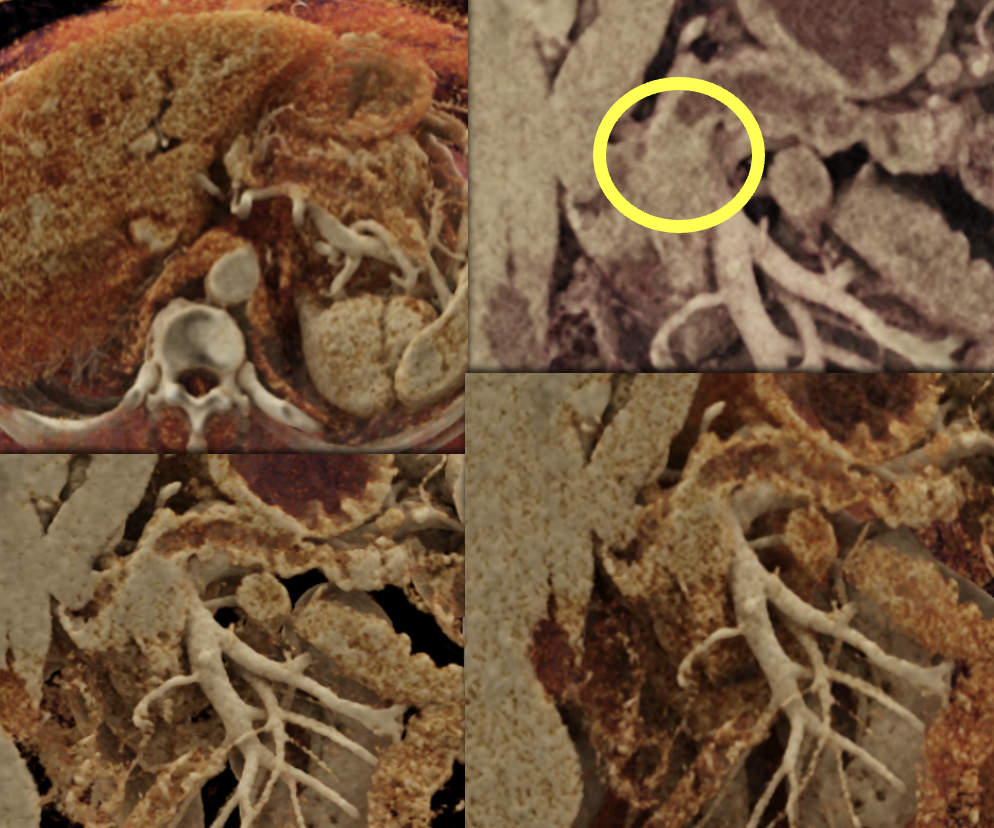 |
Future of Cinematic Rendering
|
References
|
