Primer on Distal Pancreatectomy: Indications, Surgical Approaches, Expected Post-operative Findings and Complications Every Radiologist Should KnowPrimer on Distal Pancreatectomy: Indications, Surgical Approaches, Expected Post-operative Findings and Complications Every Radiologist Should Know Franco Verde, MD |
Teaching Points
|
Objectives
|
Regional Anatomy
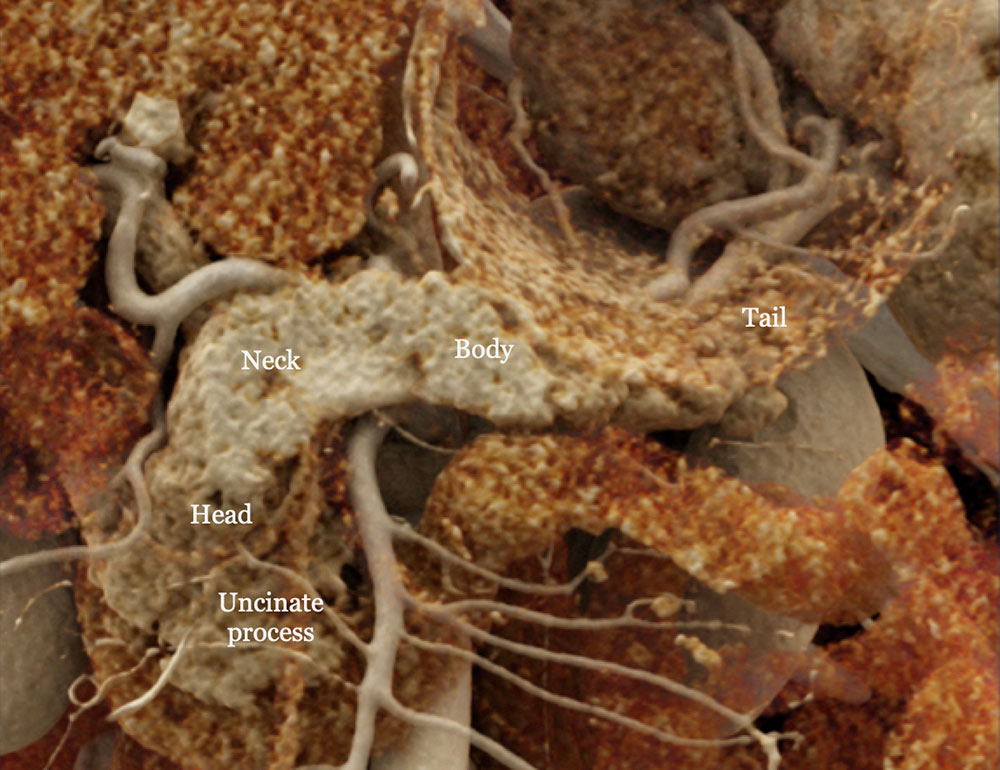 Cinematic rendering image of the normal pancreas. |
Regional Anatomy
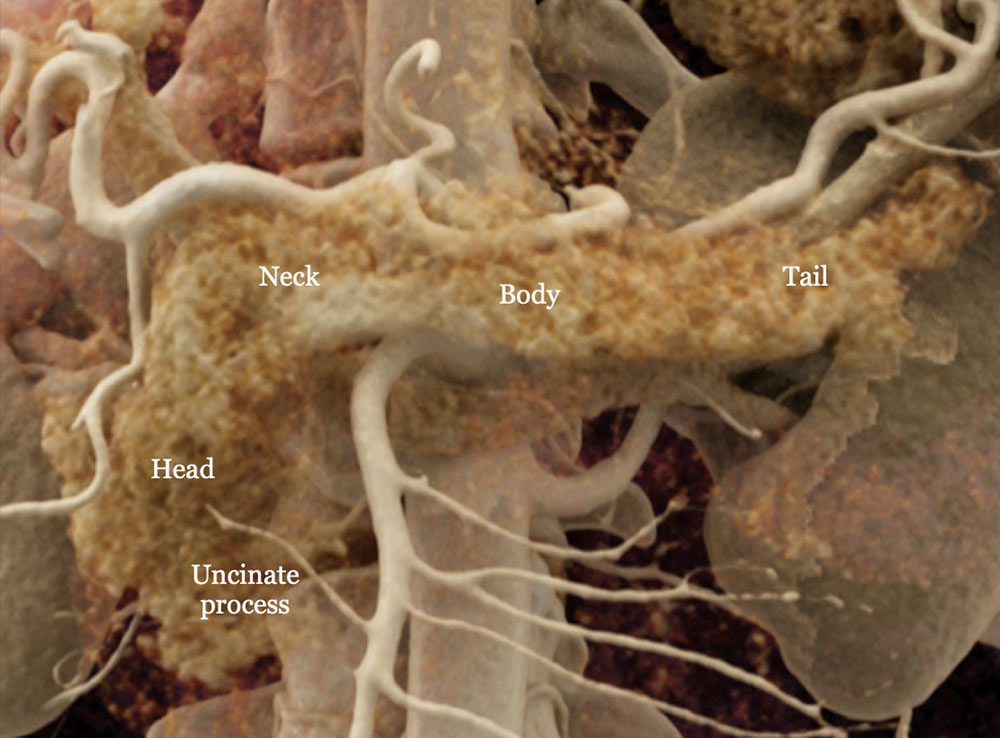 Cinematic rendering image of the normal pancreas. |
Regional Anatomy
|
Regional Anatomy: Venous Drainage
|
Indications
|
Common Indications for Distal Pancreatectomy 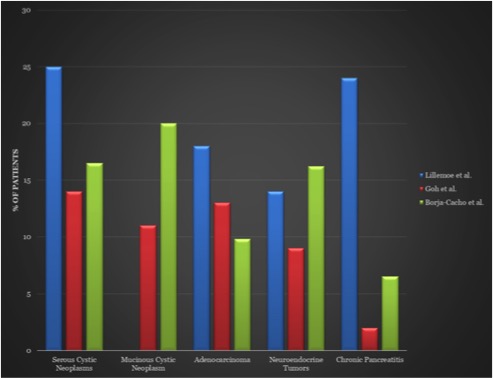 Adapted from7,8,9: 7Goh et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. Oct 2008; 143(10): 956-65. 8Lillemoe et al. Distal pancreatectomy: indication and outcomes in 235 patients. Ann Surg. May 1999; 229(5): 693-8. 9Borja-Cacho et al. Laparoscopic Distal Pancreatectomy. J Am Coll Surg. Dec 2009; 209(6): 758-65. |
Surgical Approaches: Distal Pancreatectomy
|
Surgical Approaches: Distal Pancreatectomy
|
Surgical Approaches: Distal Pancreatectomy Schematics illustrating distal pancreatectomy10. A) Through either an open incision or laparoscopically, the lesser sac is accessed and the stomach and splenic flexure of the colon are mobilized to expose the pancreas. B) Spleen and pancreatic tail are mobilized medially to expose the splenic vein. Splenic vessels are either ligated or isolated in the case of splenic preservation. C) After achieving control and hemostasis of the main pancreatic vessels, the pancreas is divided, usually just to the left of the SMV. D) Main pancreatic duct is identified and oversewn with absorbable suture, followed by suture closure of the stump. Closure may also be achieved with staples or pancreaticojejunal anastomosis. 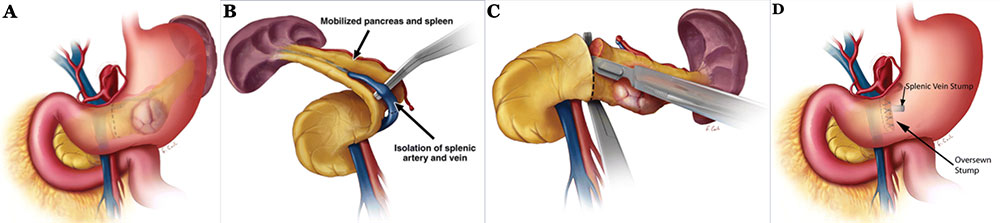 10Adapated from Wolfgang et al. Pancreatic Surgery for the Radiologist, 2011: An Illustrated Review of Classic and Newer Surgical Techniques for Pancreatic Tumor Resection. Am J Roent. 2011, 197(6): 1343-50. |
Surgical Approaches: Appleby Procedure
|
Surgical Approaches: Enucleation
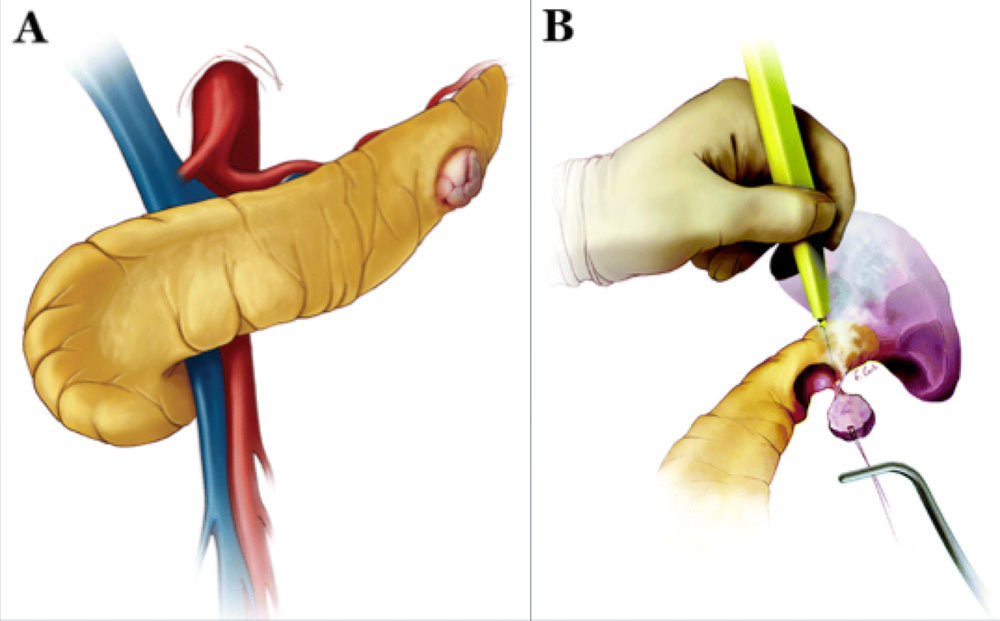 Schematics illustrating enucleation procedure10. A) The pancreas is exposed as in standard distal pancreatectomy. If the peripheral lesion is not readily identifiable, intraoperative ultrasound may be utilized. B) Lesion is excised using electrocautery or ultrasonic scalpel. If ductal injury is suspected post excision, distal pancreatectomy or pancreaticojejunostomy is performed. 10Adapated from Wolfgang et al. Pancreatic Surgery for the Radiologist, 2011: An Illustrated Review of Classic and Newer Surgical Techniques for Pancreatic Tumor Resection. Am J Roent. 2011, 197(6): 1343-1350. |
CT Technique
|
Expected Post-Operative Appearances
|
Small Fluid Collection 79 year-old male with history of pancreatic neuroendocrine tumor status post distal pancreatectomy with splenectomy. Post-operative axial (A) MDCT with coronal (B) and sagittal (C) MPRs obtained 5 days post-op for pain demonstrates a small, hypoattenuating fluid collection (gold arrows) in the pancreatectomy bed adjacent to the suture line (red arrows). Fluid collection completely resolved on 6 month surveillance scan. 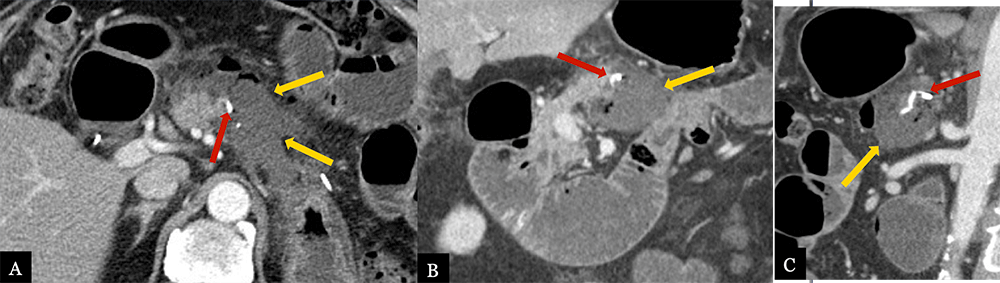 |
Small Fluid Collection 80 year-old male with history of acinar cell carcinoma status post distal pancreatectomy with splenectomy. Routine, 9 month post-op MDCT with axial (A) and coronal (B) MDCT images demonstrate a small hypoattenuating fluid collection (gold arrow) immediately adjacent to the suture line (red arrow) and pancreatic remnant. 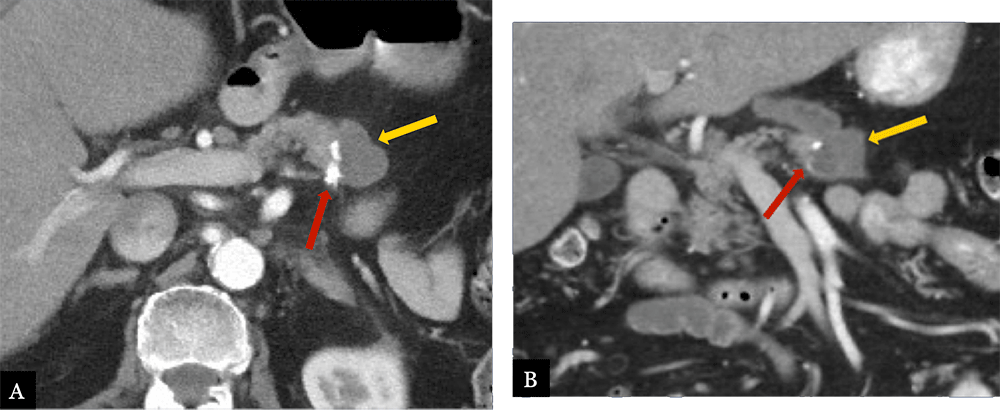 |
Omental/Fat Necrosis 63 year-old female with history of pancreatic ductal adenocarcinoma status post distal pancreatectomy and splenectomy. Routine, 6 month post-op MDCT with MPRs (A, B, D) and coronal volume-rendered image (C) demonstrate a mixed fluid and fat density collection in the pancreatectomy bed (gold arrows), compatible with fat necrosis of the lesser omentum. Note the relationship with the suture site (red arrow), however fat density and relative lack of fluid, makes fat necrosis more likely than fistula formation. 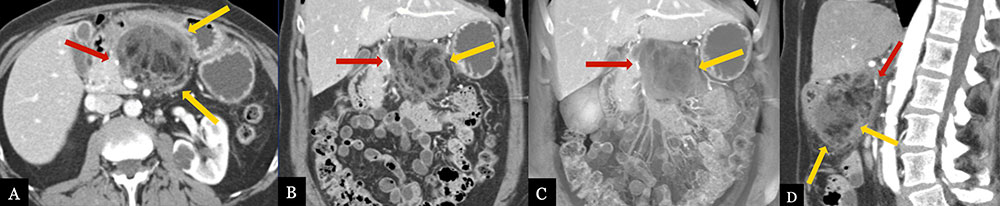 |
Omental/Fat Necrosis 70 year-old male with history of pancreatic neuroendocrine tumor status post distal pancreatectomy with splenectomy. Immediate post-operative axial (A) MDCT demonstrates ill-defined edema and fat stranding in the splenectomy bed (red arrows). Routine, surveillance axial (B) MDCT with coronal (C) volume rendered image obtained 1 year post-op demonstrates evolving, well-circumscribed hypoattenuating collection (gold arrows) with small foci of fat, most compatible with fat necrosis in the splenectomy bed. 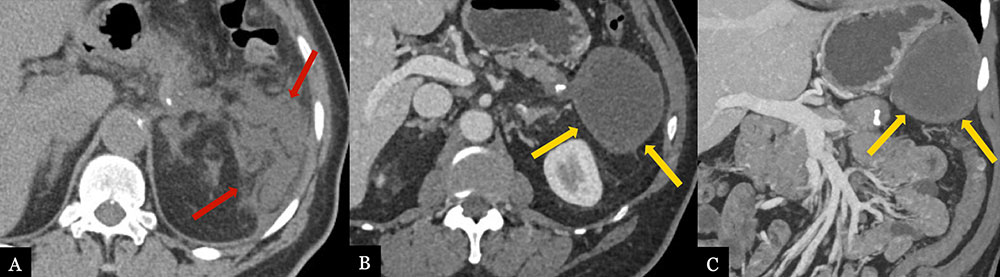 |
Post-operative Complications
|
Post-operative Complications: Pancreatic Fistula
|
Post-operative Complications: Abscess and Hemorrhage
|
Post-Operative Pancreatic Fistula 52 year-old female with history of pancreatic intraepithelial neoplasia status post distal pancreatectomy with splenectomy. Post-operative axial MDCT (A) with coronal (B) and sagittal (C) MPRs demonstrate a large fluid collection with fat stranding adjacent to the pancreatic stump (red arrow), extending to the splenectomy bed (gold arrows). 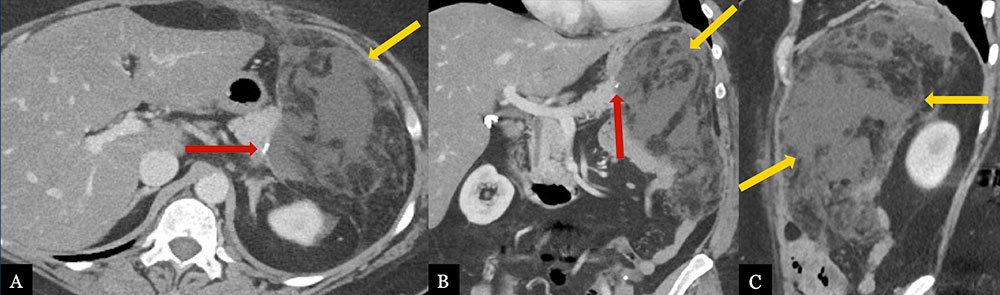 |
Pancreatic Fistula with Intra-abdominal Abscess 70 year-old male with history of pancreatic neuroendocrine tumor status post robotic, spleen preserving distal pancreatectomy. Post-operative axial MDCT (A) with coronal (B, C) and sagittal (D) MPRs demonstrate a recurrent abscess (gold arrows) adjacent to pancreatic stump (red arrow) extending to left subphrenic space, likely secondary to fistula. Note reactive gastritis (blue arrow) and gastric varices (blue arrow head) due to splenic vein thrombosis (not shown). 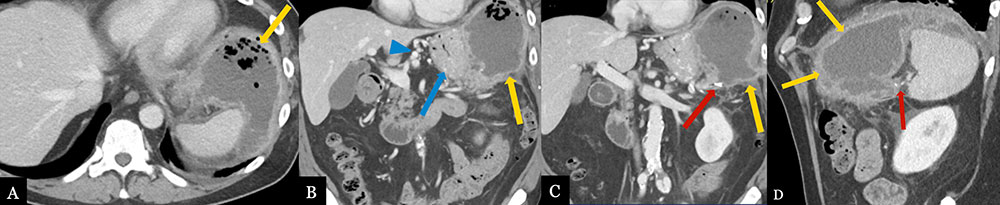 |
Lesser Sac Abscess 62 year-old male with history of inflammatory myofibroblastic tumor status post distal pancreatectomy with splenectomy. Post-operative axial (A, B) MDCT and coronal MPR (C) demonstrate abscess formation (gold arrows) in the pancreatectomy bed extending to the lesser sac. 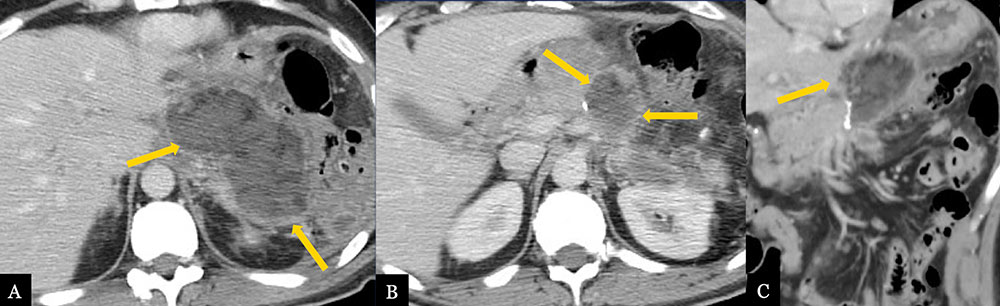 |
Intra-abdominal Abscess with Complex Gastric Fistula 59 year-old male with history of pancreatic body adenocarcinoma status post Appleby procedure. Post-operative axial MDCT (A) and coronal MPRs (B, C) demonstrate a left subphrenic abscess (gold arrows) with air and fluid tract extending to the gastric fundus concerning for fistula formation (red arrows). Fistula was confirmed on sinogram following drain placement. Note multifocal left renal infarcts (blue arrows). 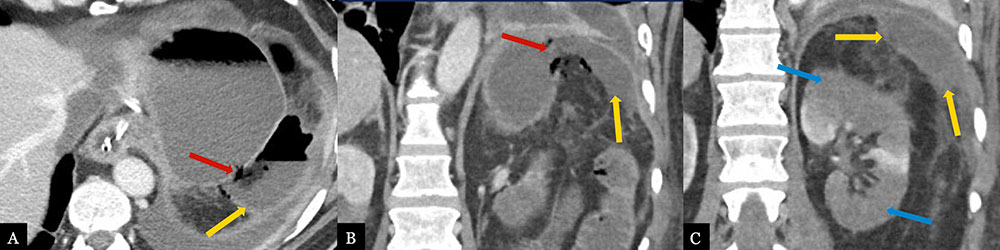 |
Large Post-operative Hematoma 31 year-old female with history of mucinous cystic neoplasm status post laparoscopic, spleen preserving distal pancreatectomy. Post-operative axial MDCT (A, B) with coronal (C) and sagittal (D) MPRs demonstrate a large, mixed density hematoma (gold arrows) in the lesser sac adjacent to the pancreatic stump (red arrow) with significant mass effect on the stomach (blue arrows). 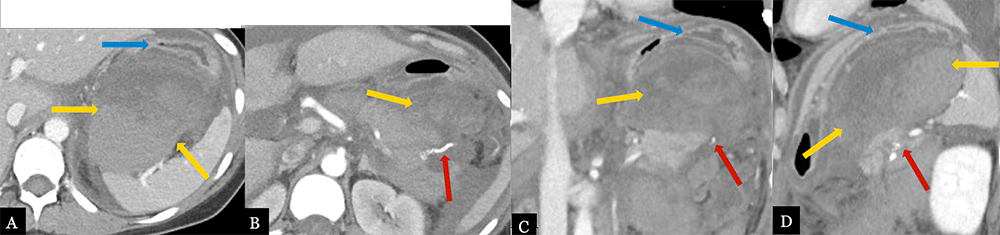 |
Acute Hemorrhage from Small GDA Branch 71 year-old male with history of pancreatic ductal adenocarcinoma status post distal pancreatectomy with splenectomy and portal vein resection. Acute, post-operative GI bleeding prompted CTA. Axial (A), arterial phase MDCT images with maximum intensity projection images (B) and coronal MPRs (C) demonstrate active contrast extravasation from a small branch off the GDA (gold arrows). Active hemorrhage (red arrows) was confirmed on celiac arteriogram (D). 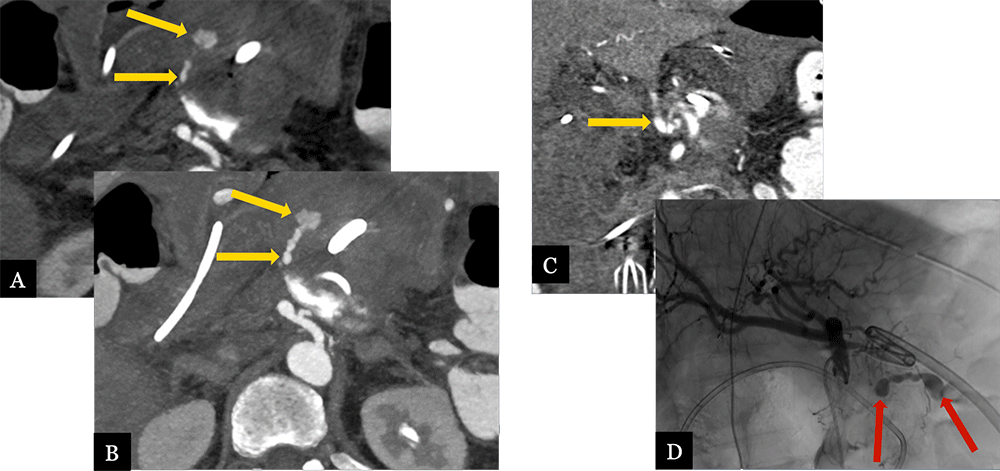 |
Acute, Perioperative Hemorrhage from Splenic Artery Stump 50 year-old female with history of pancreatic neuroendocrine tumor status post laparoscopic distal pancreatectomy with splenectomy. Post-operative MDCT with axial arterial (A) and venous (B) phase MIPs and coronal MPR (C) demonstrate contrast extravasation (gold arrows) from the splenic artery stump, indicating active hemorrhage. Note the large hematoma (red arrows) in the lesser sac. 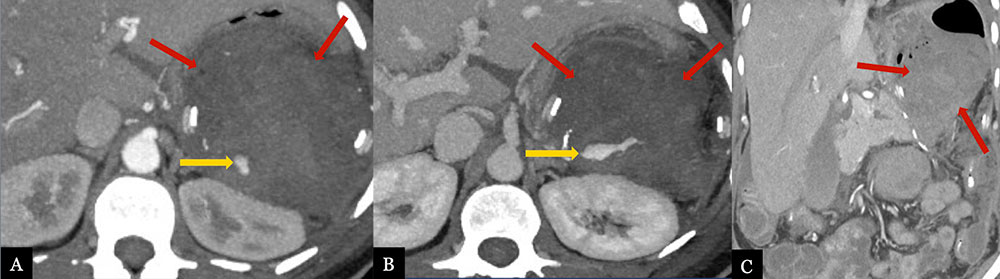 |
Large Splenic Artery Pseudoaneurysm 62 year-old male with history of serous cystadenoma status post spleen preserving distal pancreatectomy. Patient presented with acute GI bleed and abdominal pain approximately 6 months post-op. Axial MDCT (A) with axial MIP images (B), coronal (C) and sagittal MPRs (D) demonstrate a large, 9 cm, partially thrombosed pseudoaneurysm arising from the splenic artery (gold arrows). Note hyperattenuating hematoma anterior and inferior to the PSA (red arrows). 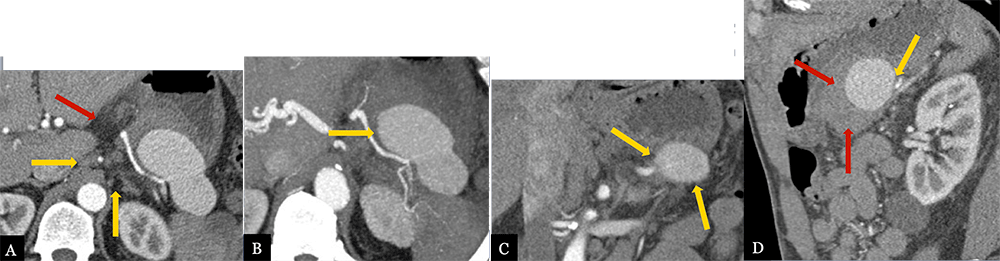 |
Take-Home Points
|
References
|
References
|
References
|
References
Acknowledgements
|
