Calcified Pancreatic and Peripancreatic Masses: Common to Uncommon PathologiesCalcified Pancreatic and Peripancreatic Masses: Common to Uncommon Pathologies Franco Verde, MD; Elliot K Fishman, MD |
Disclosures
|
Introduction
|
Outline
|
Protocol Optimization Recommended 64 MDCT protocol
|
Common pancreatic masses that may calcify: Ductal adenocarcinoma
|
49 year old woman with pancreatic mass. IV contrast enhanced CT with axial arterial phase (A), axial venous (B), coronal arterial (C) and coronal thick-slab MIP venous (D) images. Obstructing pancreatic mass seen with ill-defined hypoattenuation and coarse calcification (white arrows). Peripancreatic necrotic adenopathy is present (gold arrow). A common bile duct stent is in place (arrow head). ERCP brushing revealed adenocarcinoma of pancreatic primary. 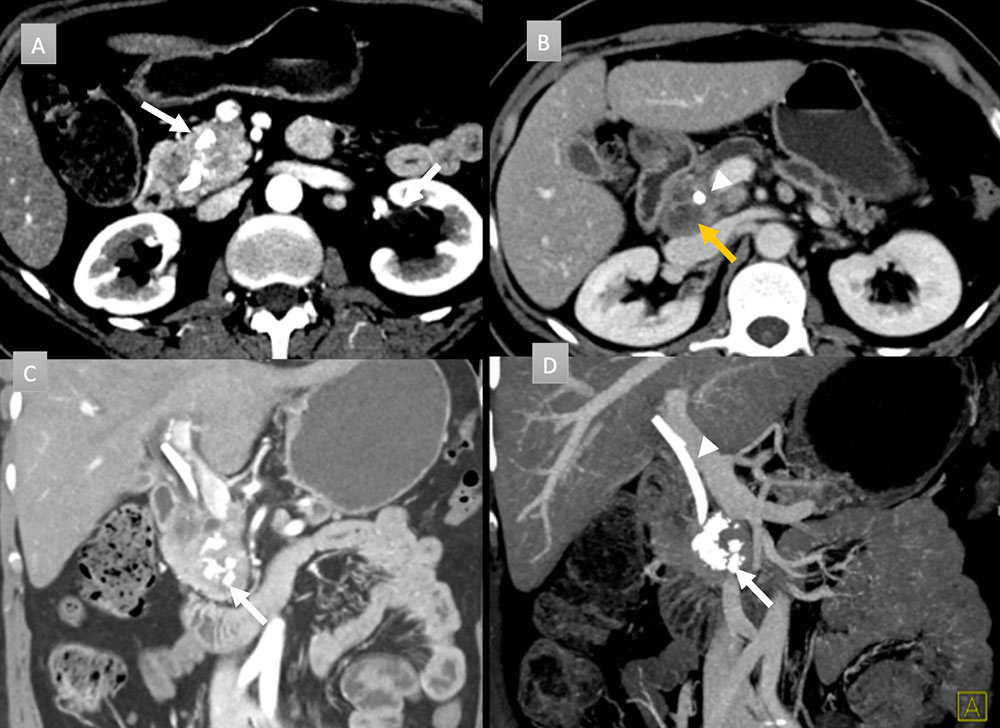 |
Uncommon masses that may calcify: Neuroendocrine tumors
|
58 year old man with pancreatic mass. IV contrast enhanced CT in axial arterial (A) and portal venous phases (B and C) demonstrate a cluster of coarse calcifications in the pancreatic tail (white arrows) with adenopathy (short white arrows) and hepatic metastatic disease (black arrows). Coronal thick slab MIP (D) well depict the calcifications throughout the mass and punctate calcifications in the metastatic gastrohepatic and peripancreatic adenopathy (arrow heads). Liver biopsy revealed a well-differentiated neuroendocrine tumor. Patient was not a surgical candidate and received chemotherapy. 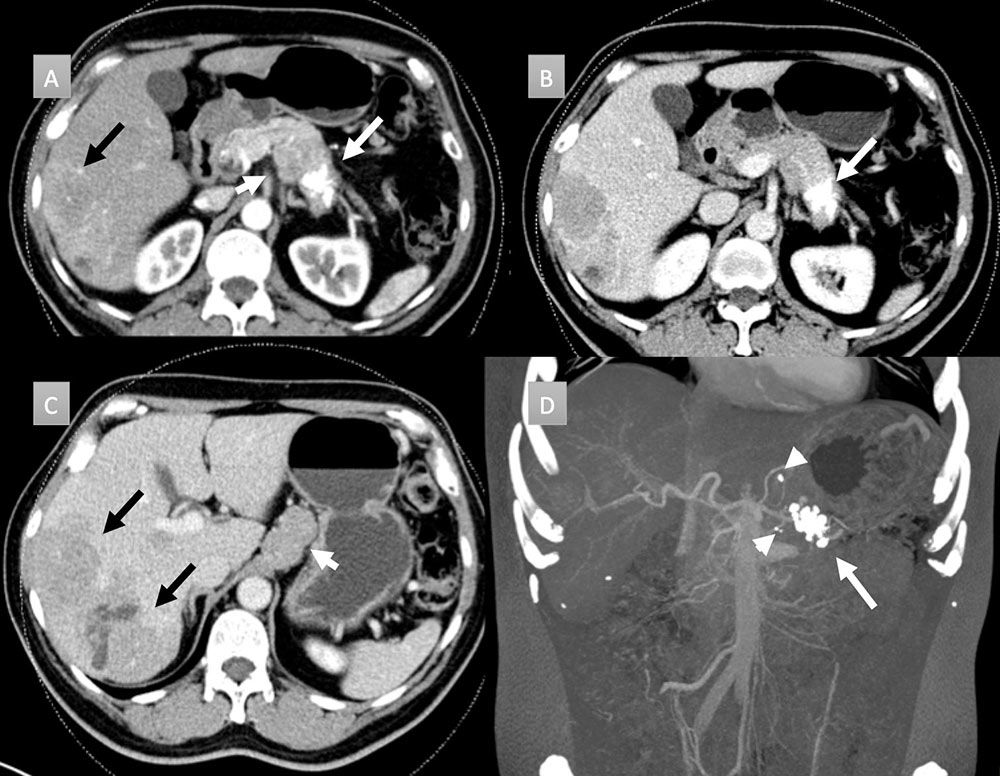 |
67 year old woman with pancreatic mass. IV contrast enhanced CT in axial and coronal arterial (A &C) and portal venous phases (B) demonstrate a 6 cm heterogenous peripherally enhancing mass with a punctate calcification (arrow). Coronal thick slab MIP (D) demonstrates the vascularity of the mass with punctate calcification (arrow). FNA demonstrated a well-differentiated neuroendocrine tumor. Patient was not a surgical candidate and received chemoradiation. 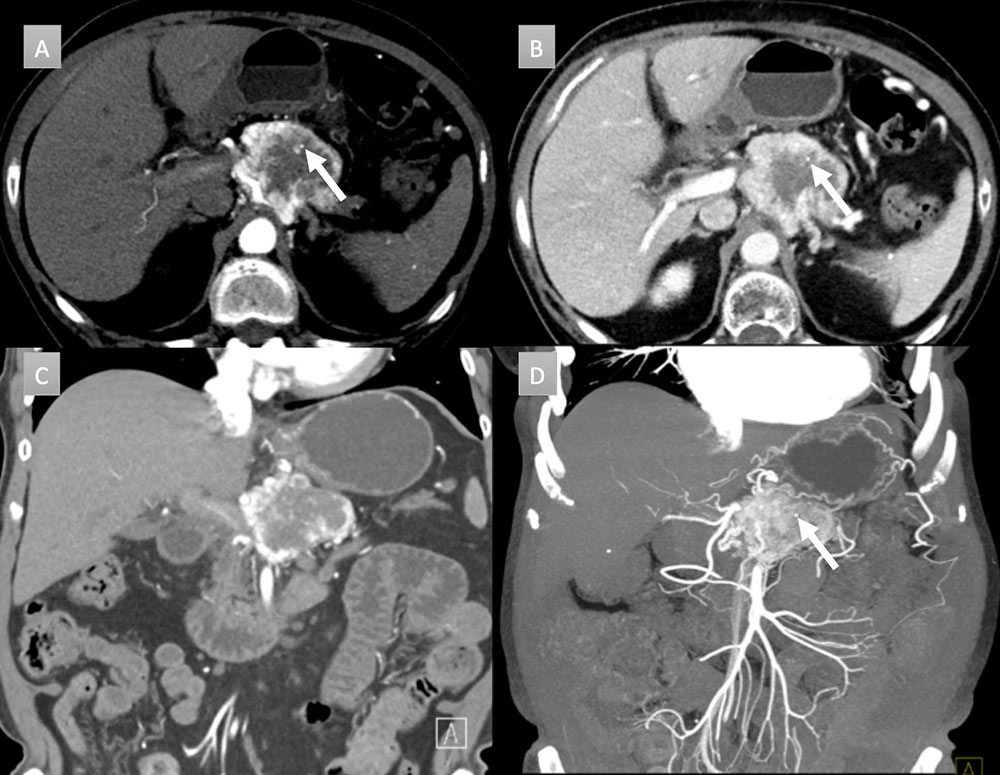 |
Uncommon masses that may calcify: Serous cystadenoma
|
71 year old woman with symptomatic pancreatic mass. IV contrast enhanced CT in axial arterial (A) and portal venous phases (B) demonstrate a 8.6 x 6 cm hypovascular mass with microcystic appearance and prominent rim vascularity. Central cluster of coarse calcifications are present (arrows). Patient was a surgical candidate and underwent Whipple procedure demonstrating a serous cystadenoma without infiltrating carcinoma. 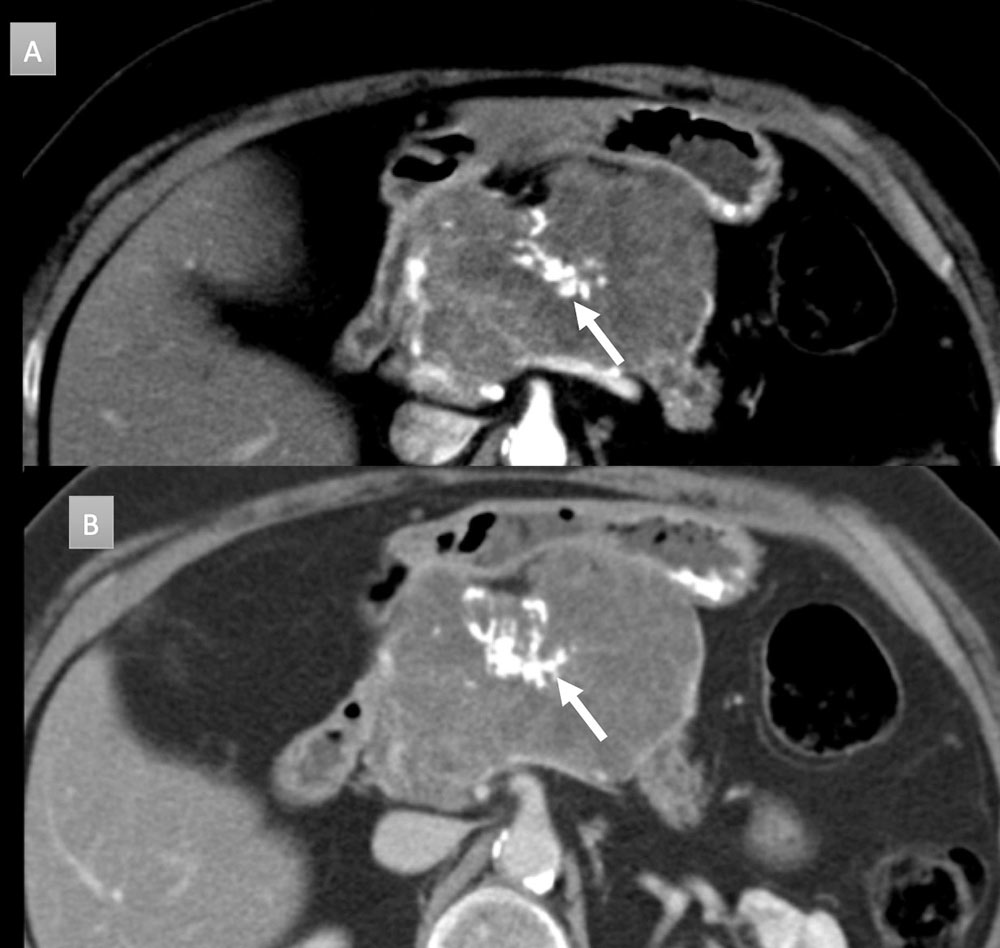 |
Uncommon masses that may calcify: Solid pseudopapillary epithelial neoplasm (SPEN)
|
30 year old woman with pancreatic mass. IV contrast enhanced CT in axial arterial (A) and venous (B) phases with coronal thick slab MIP (C) and 3D rendering (D) demonstrate a large pancreatic mass with rim interrupted calcifications (arrows). Mass demonstrates soft tissue and cystic components. MIP imaging fully depicts all the calcification in one image (black arrows). Mass was resected and revealed a solid pseudopapillary epithelial neoplasm. 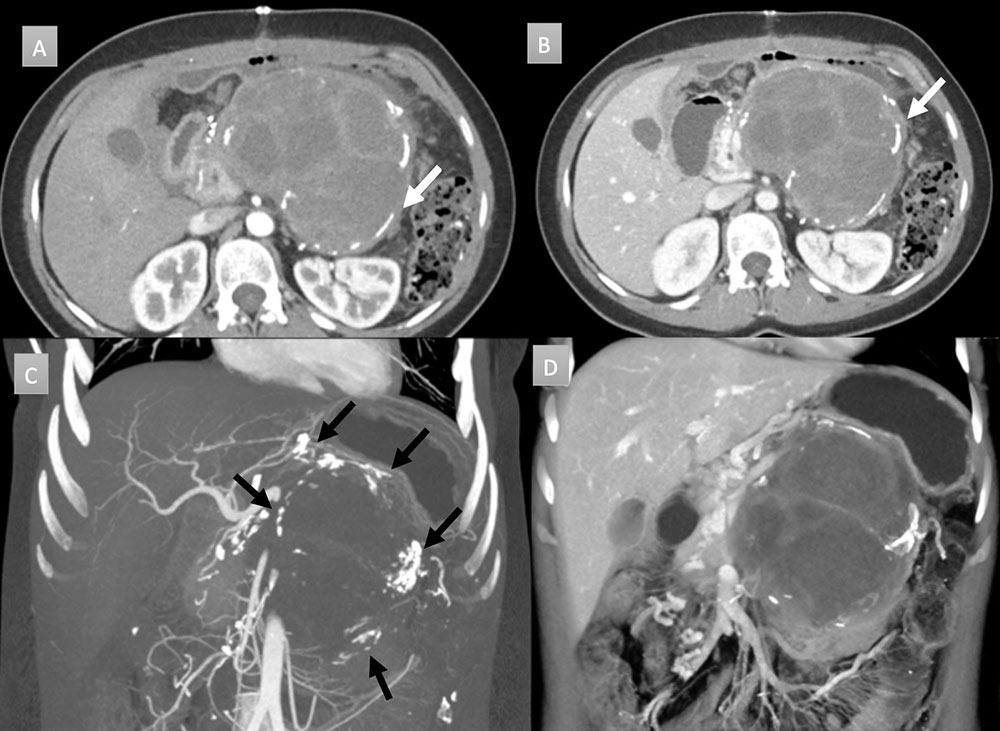 |
Uncommon masses that may calcify: Intraductal papillary mucinous tumor
|
68 year old man with pancreatic mass. IV contrast enhanced CT in axial arterial phase (A) and axial, coronal, and coronal 3D portal venous phase (B, C, and D) demonstrates 1.5 x 2 cm pancreatic head cystic mass with eccentric cluster of coarse calcifications (arrow). Additional punctate calcification seen cranial to larger cluster (figure A arrow). EUS/FNA was performed demonstrating mucinous features and communication with pancreatic duct, consistent with an intraductal papillary mucinous tumor. 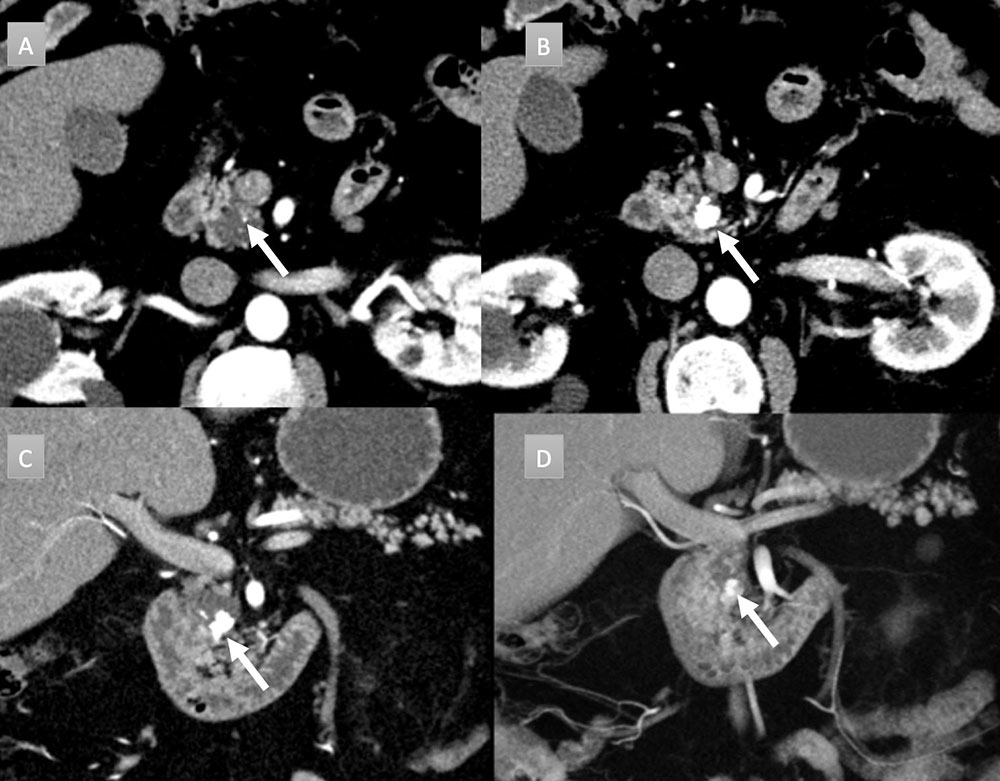 |
Uncommon masses that may calcify: Colloid carcinoma arising from IPMN
|
69 year old man with pancreatic mass. IV contrast enhanced CT in axial arterial phase (A) and in axial, coronal, and MIP portal venous phase (B, C, and D) demonstrates massive dilation and sacculation of the main duct with extensive mural nodularity (arrows heads) and areas of coarse calcification (arrows). Partially imaged pancreatic head is enlarged with acute pancreatitis (not labelled). Patient underwent distal pancreatectomy revealing colloid carcinoma arising from IPMN. Nodal and omental metastases were present (not shown). 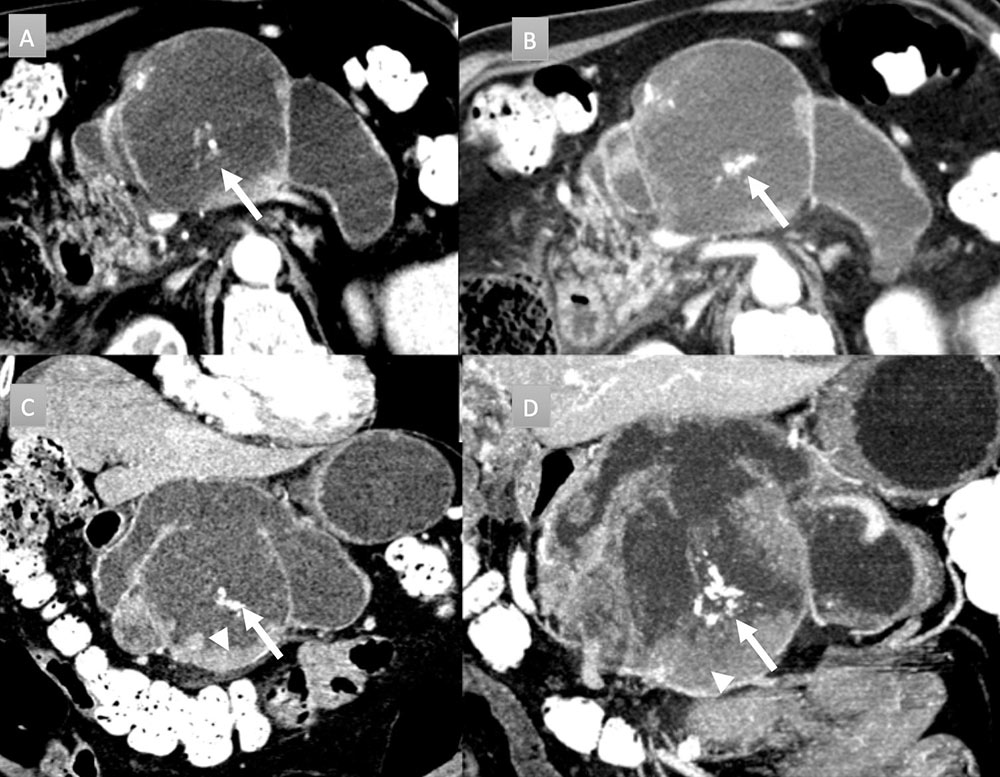 |
Uncommon masses that may calcify: Mucinous cystic neoplasm (MCN)
|
48 year old woman with pancreatic mass. IV contrast enhanced CT in axial arterial phase (A) axial venous (B), coronal arterial (C), and coronal venous (D) phases. Well demarcated, 3 cm, partially exophytic, cystic mass, arising from the pancreatic body. No internal septation or debris present. Two punctate calcifications are present (arrows). No communication with duct was noted. Patient underwent EUS-FNA revealing very high CEA levels, consistent with a mucinous cystic neoplasm. 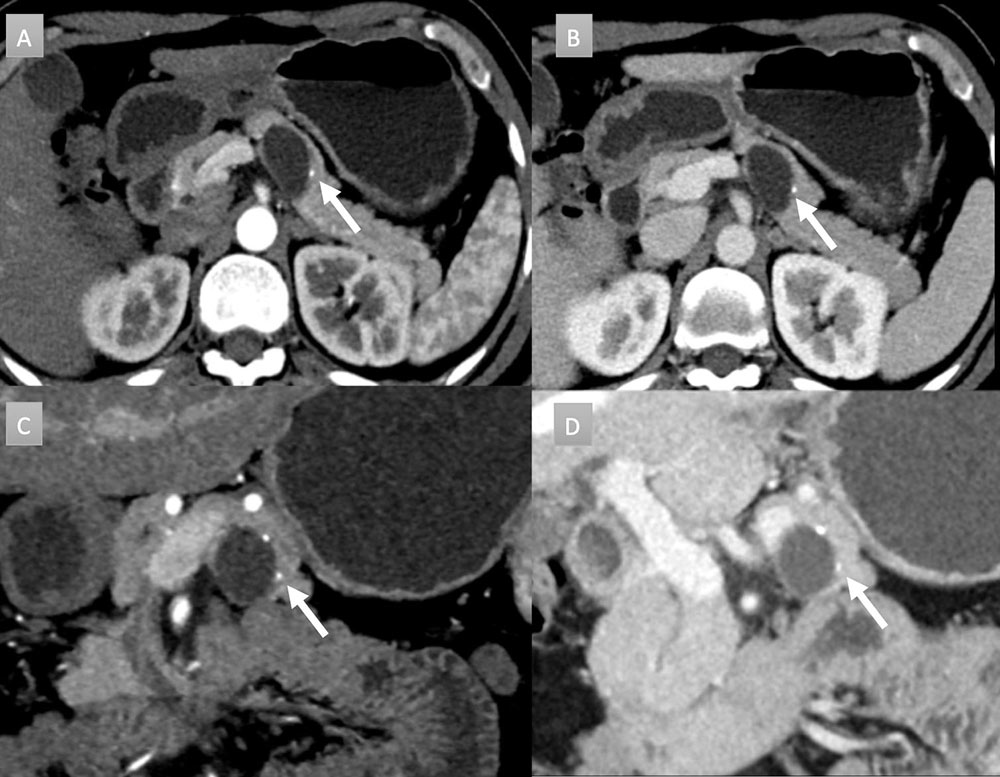 |
Uncommon masses that may calcify: Lymphoepithelial Cyst
|
66 year old man with peripancreatic mass. IV contrast enhanced CT with selected axial arterial (A), axial venous (B), coronal arterial (C) and coronal thick-slab MIP arterial (D) images. Large round cystic mass arising near the tail of the pancreas, measuring 14 x 12 x 15 with mass displacement of the stomach. Note punctate and coarse calcifications along the wall (arrows). Resection demonstrated lymphoepithelial cyst without evidence of carcinoma. 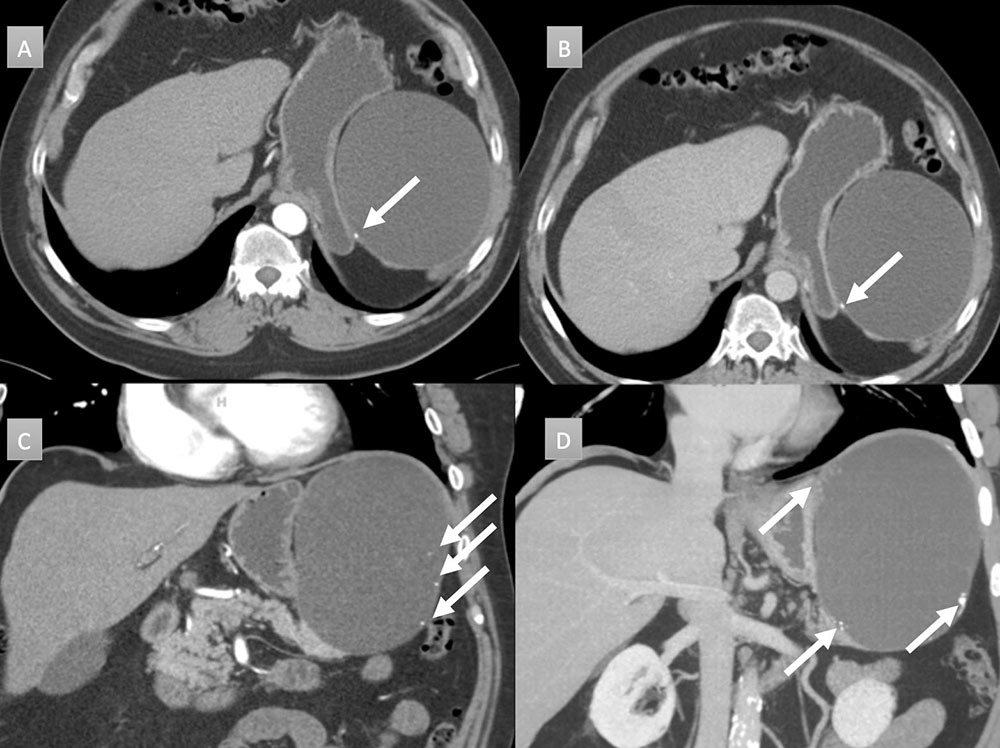 |
Peripancreatic masses that can calcify: Duodenal adenocarcinoma
|
65 year old man with peripancreatic mass. IV contrast enhanced CT with selected axial arterial (A) and portal venous phases axial (B), coronal (C) and coronal 3D (D) images. Cluster of calcifications noted near the uncinate process abutting third portion duodenum (white arrows). Subtle thickening of the 2nd portion duodenum near the ampulla also seen, best depicted on 3D image (gold arrow). Histology revealed a 4.1 cm mucinous adenocarcinoma with pancreatic invasion and regional metastatic adenopathy (not shown). 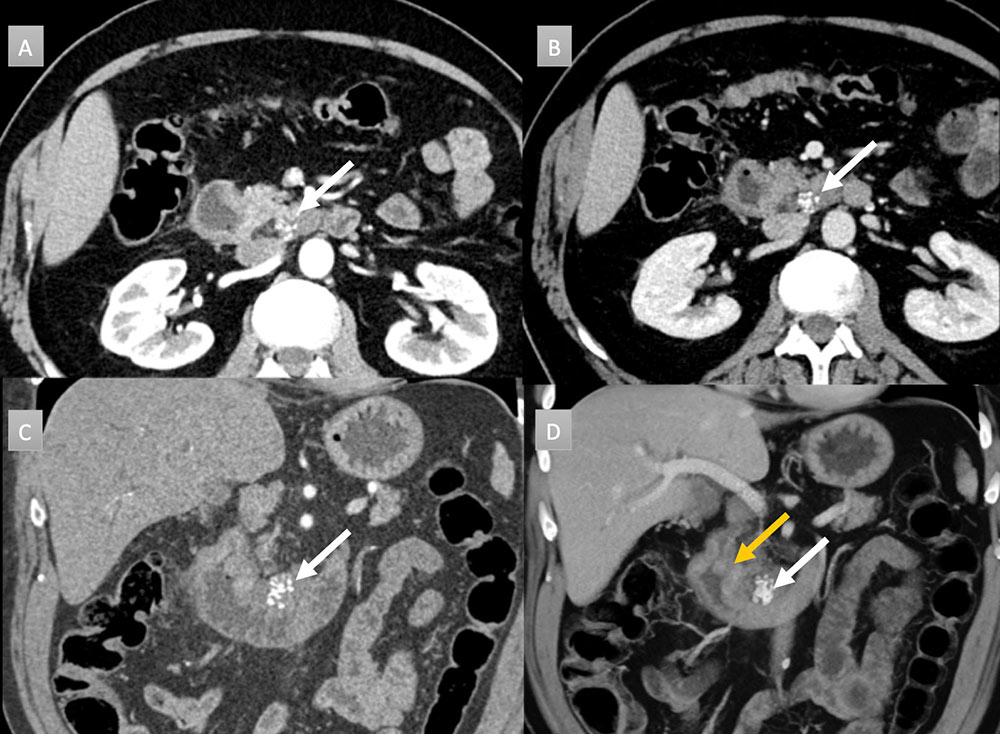 |
Peripancreatic masses that can calcify: Pancreatic Pseudocyst
|
42 year old woman with peripancreatic mass. IV contrast enhanced CT in axial arterial phase (A) demonstrates a 4cm finely rim calcified mass with central hypoattenuation (white arrow). Available prior from 7 years ago (B) demonstrates acute interstitial edematous pancreatitis with pancreatic head enlargement (black arrow) and extensive acute pancreatic fluid (yellow arrows). Current findings are consistent with a peripancreatic pseudocyst. No intervention was performed. 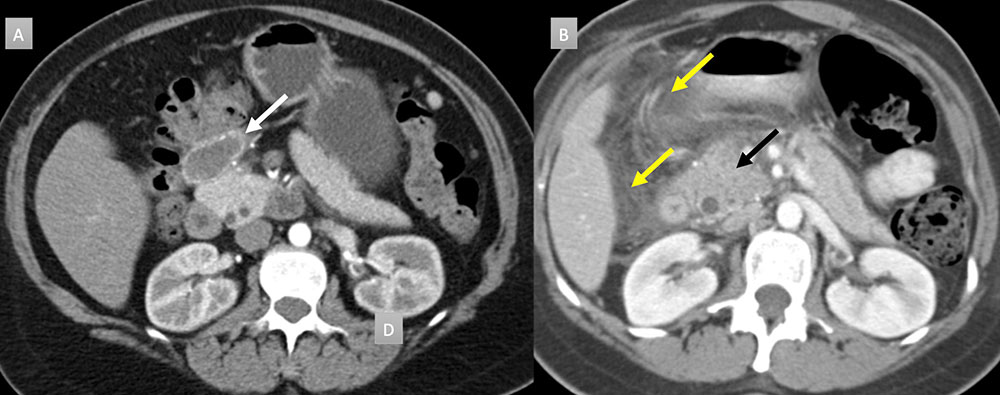 |
Peripancreatic masses that can calcify: Desmoplastic Small Round Cell Tumor (DSRCT)
|
22 year old man with history of metastatic desmoplastic small cell tumor. IV contrast enhanced CT with selected axial arterial (A) and portal venous phases axial (B), coronal (C) and coronal MIP (D) images. Evidence of extensive metastatic disease is seen on all images as numerous cystic and hypodense masses throughout the liver (*). A large peripancreatic heterogeneous hypodense mass is seen with central scattered coarse calcifications (arrows). Note distal pancreatic parenchymal atrophy and duct dilation related to mass compression (gold arrow). 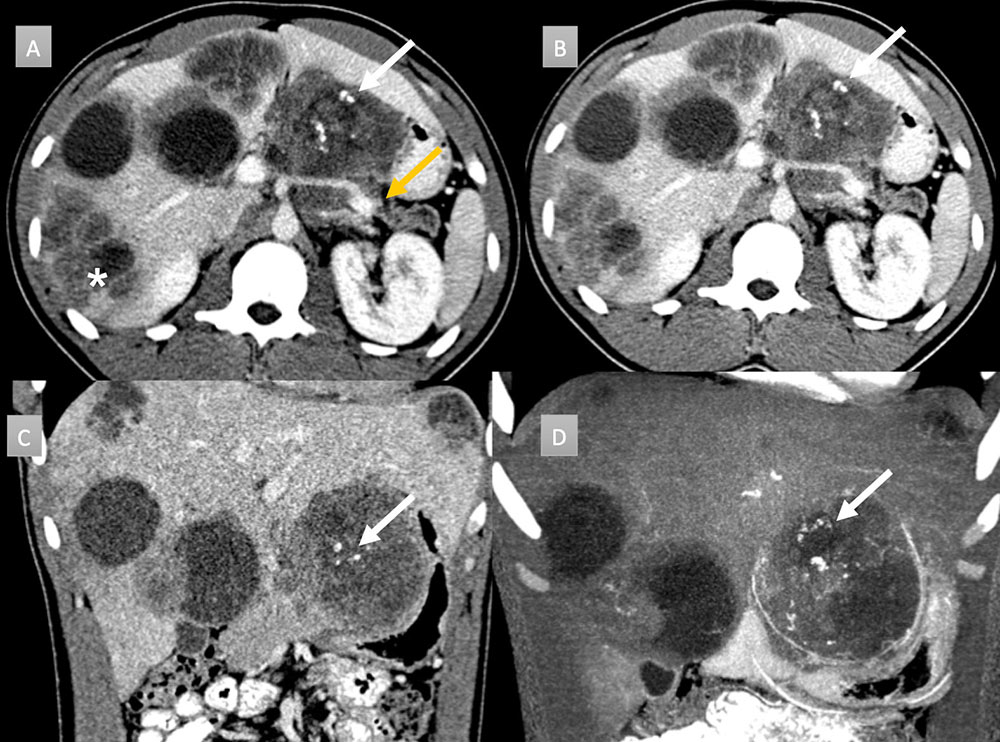 |
Peripancreatic masses that can calcify: Pitfall - Chronic pancreatitis
|
64 year old man with abdominal pain IV contrast enhanced CT with selected axial arterial (A,B,C) and venous oblique coronal (D) images. Evidence of chronic pancreatitis seen with parenchymal atrophy, punctate parenchymal calcifications (short arrows) and 8 mm duct dilation. Intraductal stone present (gold arrow). Additional ill-defined low attenuation anterior to pancreatic neck and head (long arrows) concerning for adenocarcinoma. EUS-FNA performed demonstrating only inflammation. 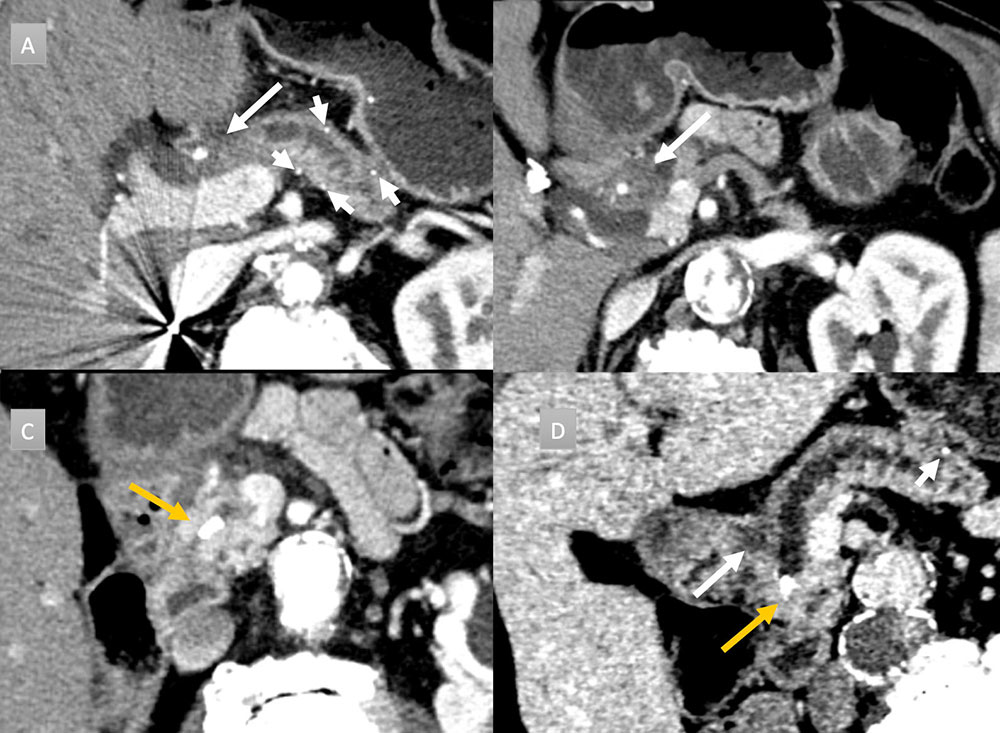 |
Summary
|
References
|
