CT after Pancreaticoduodenectomy with Portal Vein and/or Superior Mesenteric Vein Reconstruction: Review of Current Surgical Techniques and Associated Post Surgical Imaging FindingsCT after Pancreaticoduodenectomy with Portal Vein and/or Superior Mesenteric Vein Reconstruction: Review of Current Surgical Techniques and Associated Post Surgical Imaging Findings Elliot K. Fishman, M.D. The Russell H. Morgan Department of Radiology and Radiological Science The Johns Hopkins Medical Institutions Baltimore, Maryland |
Introduction
|
Purpose
|
Materials and Methods: Patients
|
Materials and Methods: Patients
|
Materials and Methods: Patients
|
Materials and Methods: Post-PD CT protocol
|
Materials and Methods: Surgical Analysis
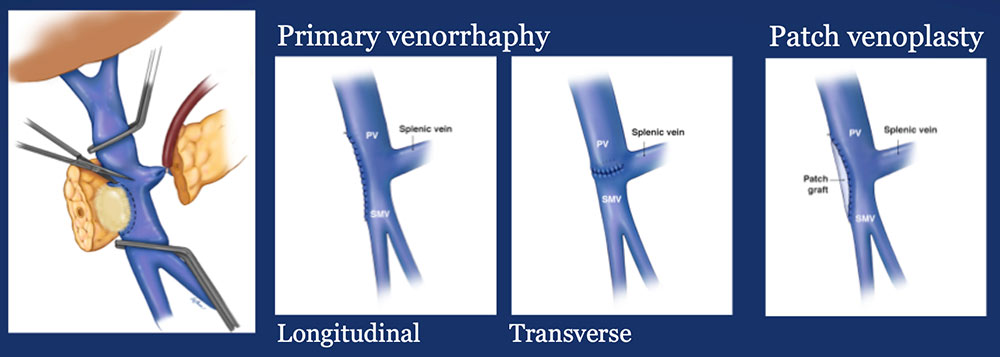 |
Materials and Methods: Surgical Analysis
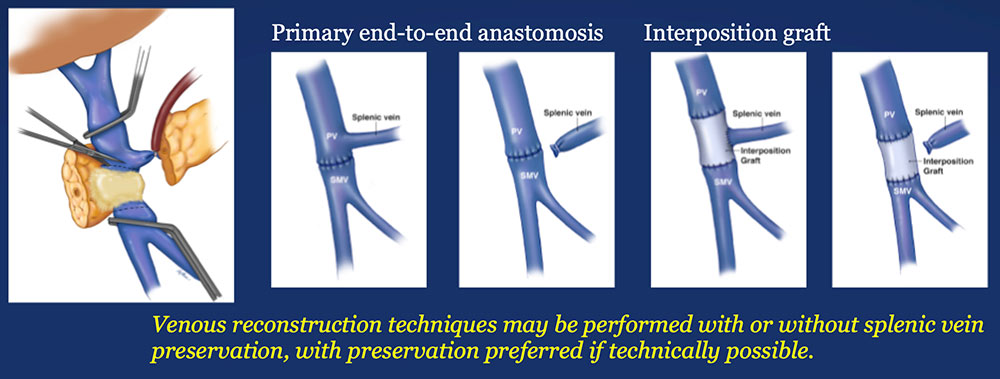 |
Results – PVR techniques  Primary reconstruction (without the use of a conduit) was performed in 90% of the cases. Interposition grafts most commonly included the IJ or renal vein. PTFE grafts are rarely used because of association with thrombosis. |
Results – PVR techniques PV-SMV findings: Four patterns of PV-SMV changes are seen on CT after PVR 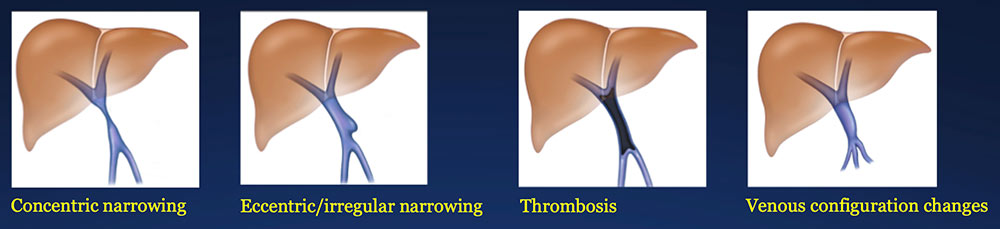 Perivenous findings: Two patterns of perivenous changes are seen on CT after PVR 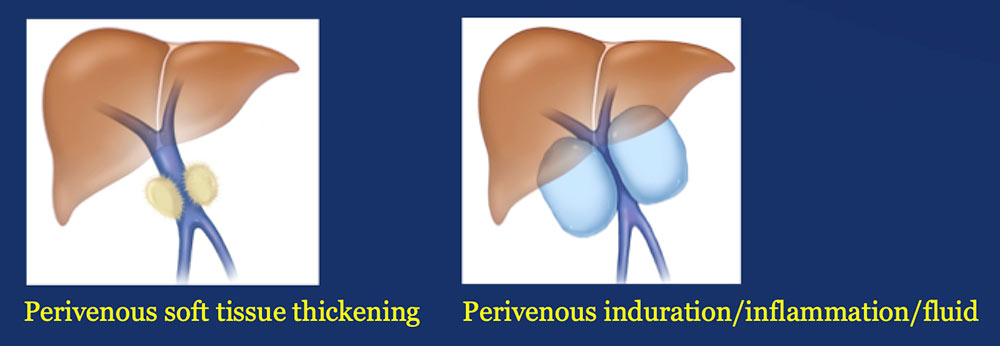 |
PV-SMV: Venous narrowing on CT after PVR 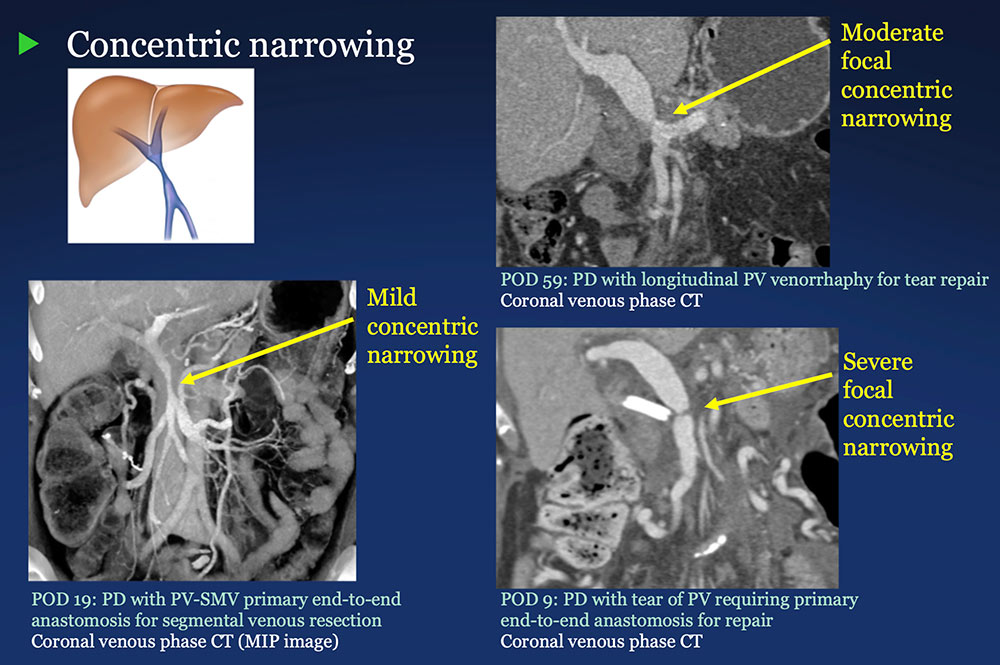 |
PV-SMV: Venous narrowing on CT after PVR 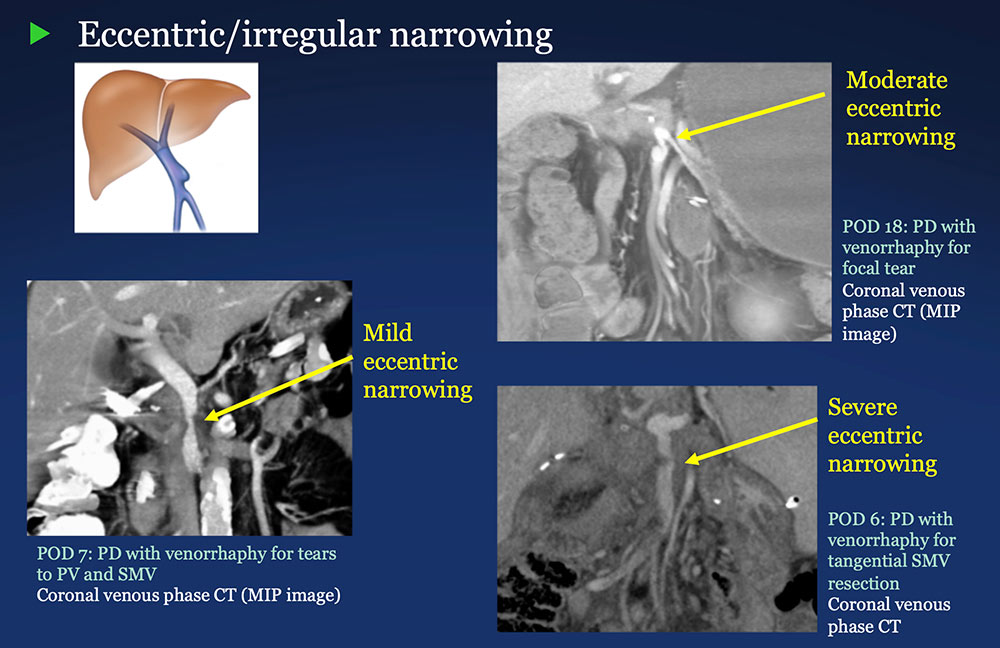 |
PV-SMV: Venous narrowing on CT after PVR 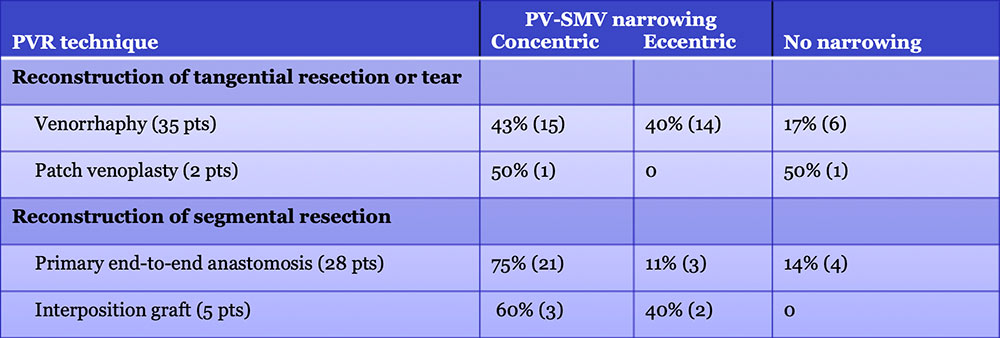 PVR procedures were associated with post op PV-SMV narrowing in 84% of patients, ranging from mild to severe. 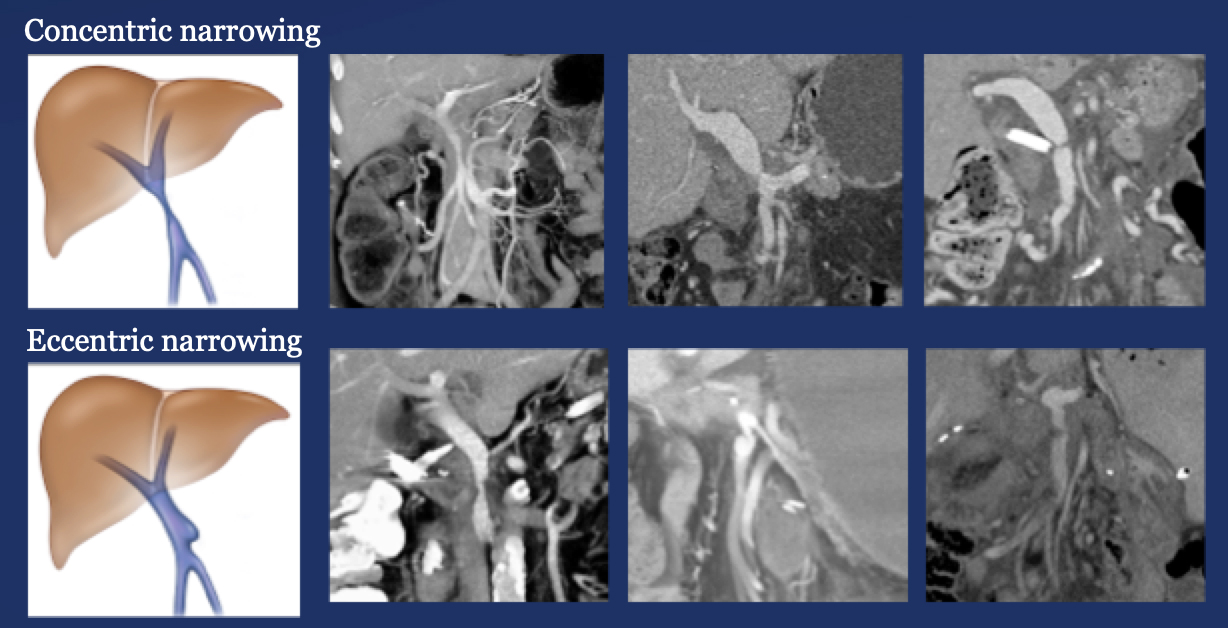 |
PV-SMV: Venous thrombosis on CT after PVR 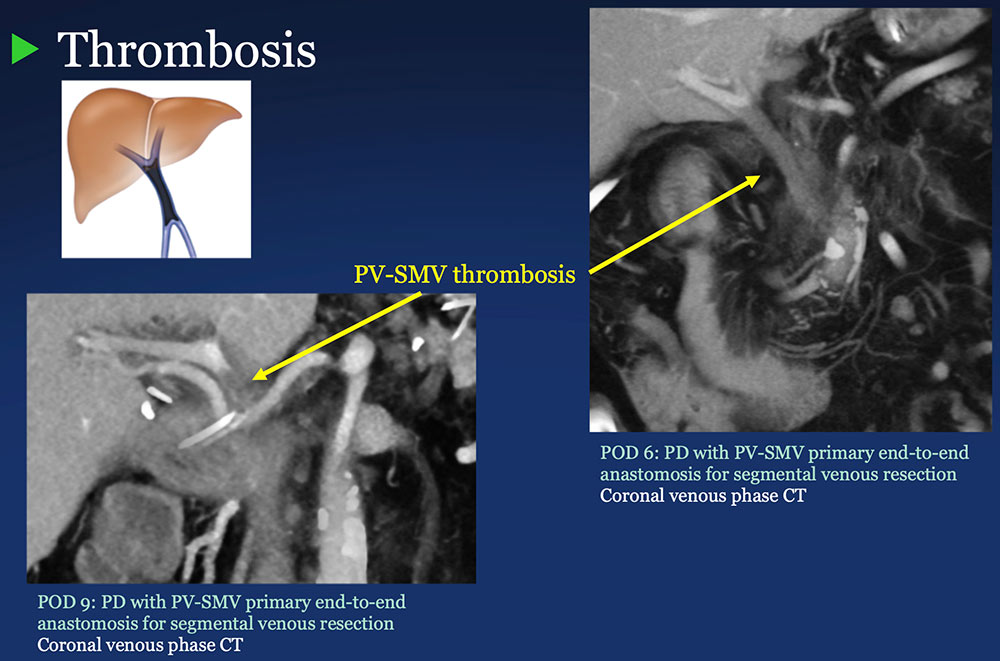 |
PV-SMV: Venous thrombosis on CT after PVR 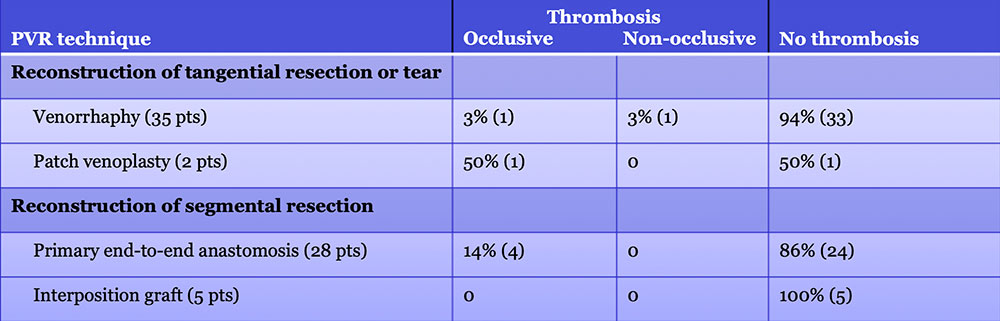 Occlusive thrombosis was seen in 9% of cases after PVR.  |
Perivenous space: Perivenous ST thickening on CT after PVR 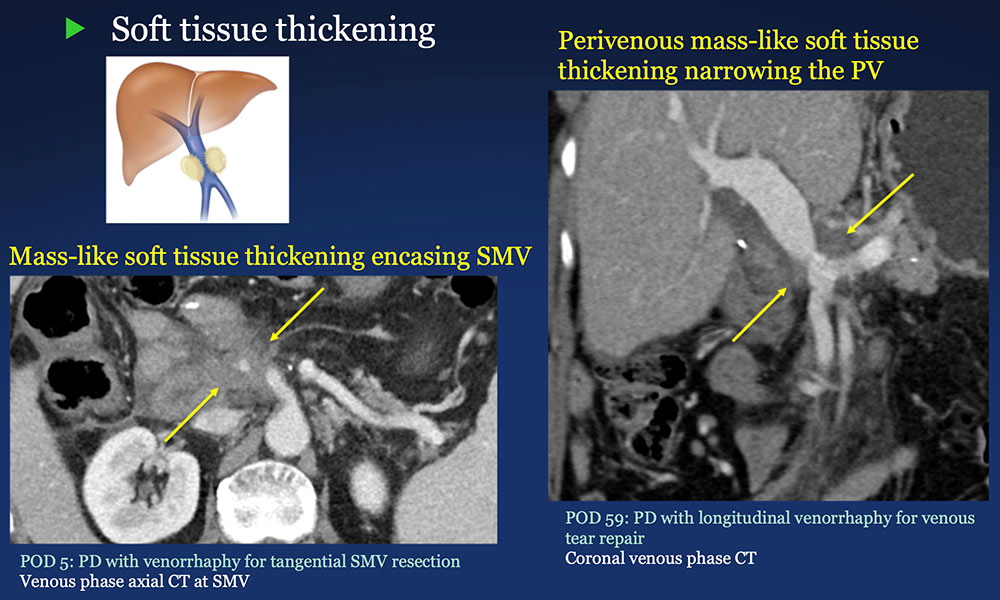 |
Perivenous space: Perivenous ST thickening on CT after PVR 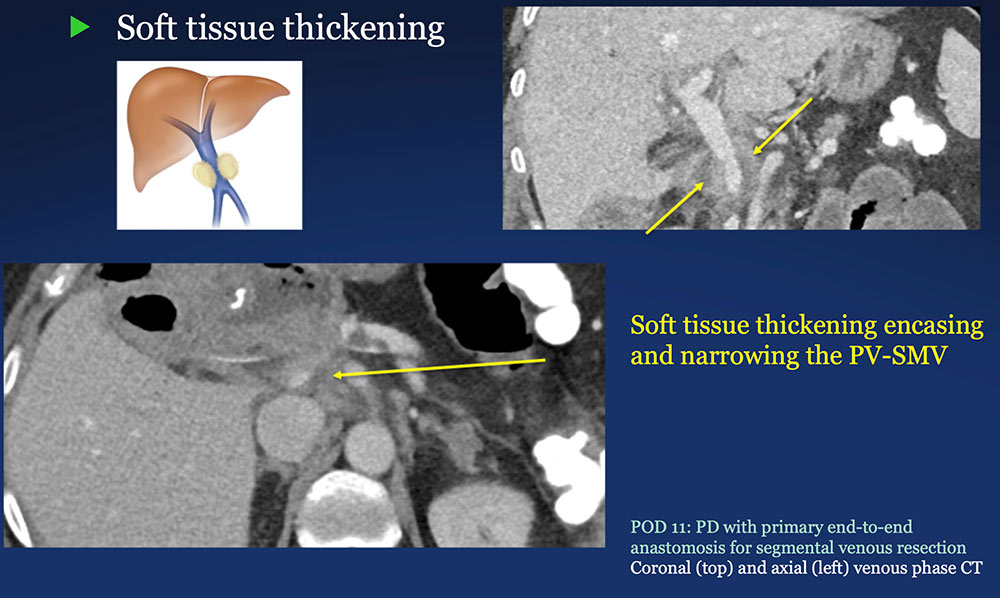 |
Perivenous space: Induration/inflammation/fluid on CT after PVR 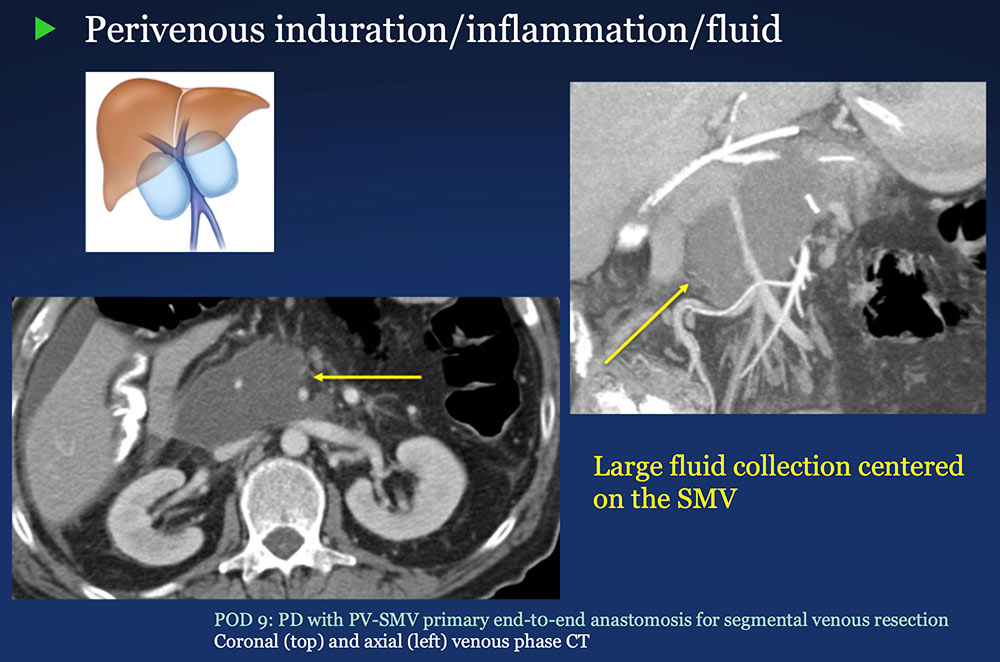 |
Perivenous space: Induration/inflammation/fluid on CT after PVR 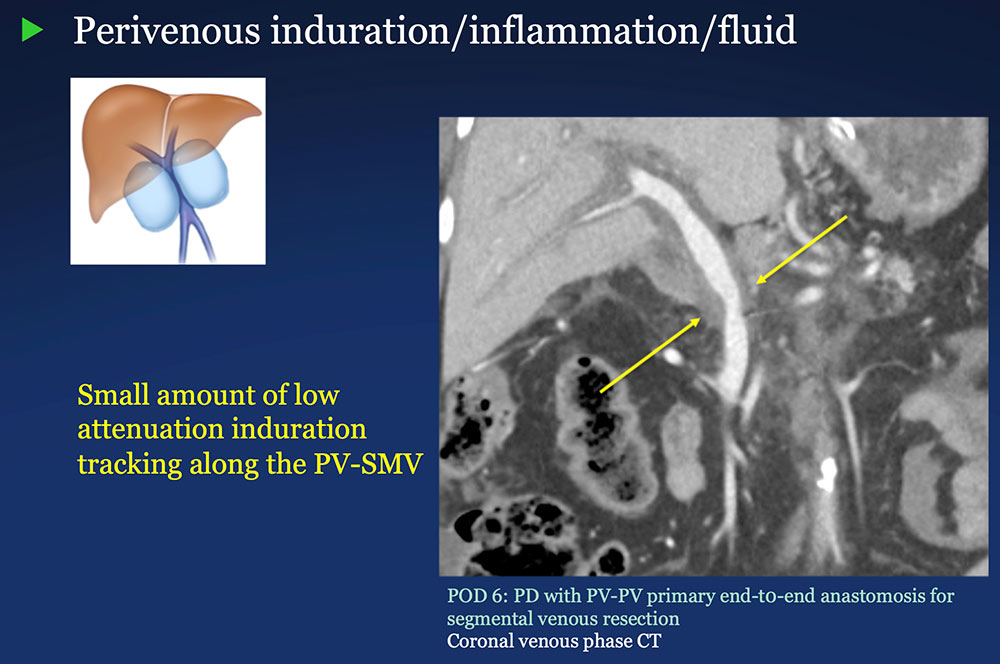 |
Perivenous space: CT Findings after PVR 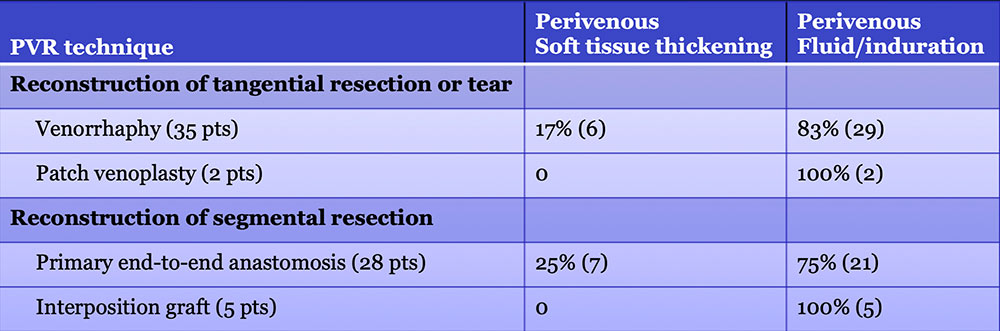 Mass-like perivenous soft tissue thickening was seen in 19% of patients after PVR, and was limited to patients with primary reconstructions, however, the number of cases of graft reconstruction was small in our sample. 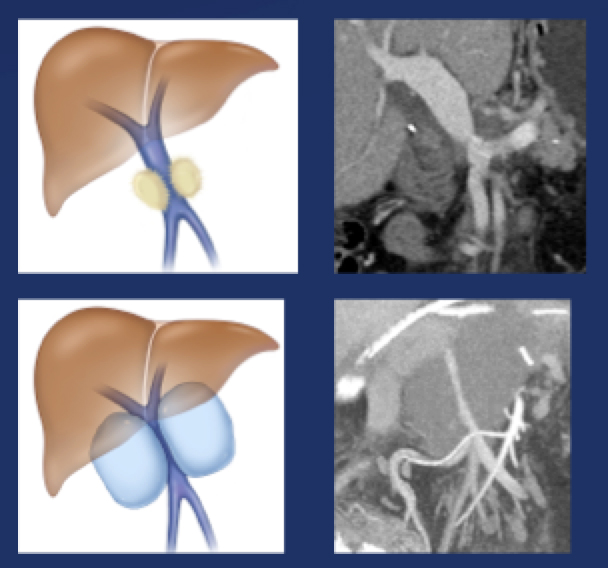 |
Results – Summary The CT appearance of the PV-SMV after PVR includes four patterns 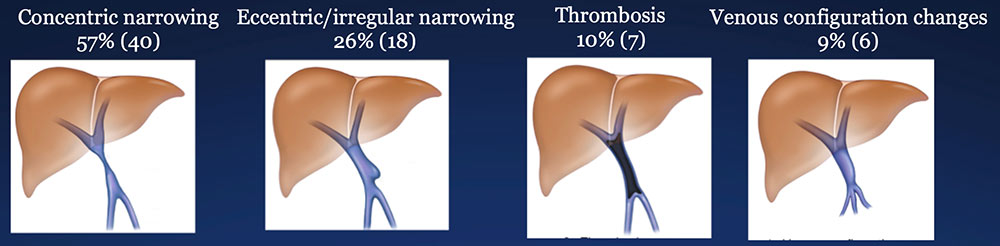 The PV-SMV patterns are not mutually exclusive; some patients had findings in more than one category. |
Results – Summary The CT appearance of the PV-SMV after PVR includes four patterns  The PV-SMV patterns are not mutually exclusive; some patients had findings in more than one category. 91% of the patients had PV-SMV changes that can also be seen with recurrent pancreatic cancer |
Results – Summary The CT appearance of the perivenous space after PVR includes two patterns: 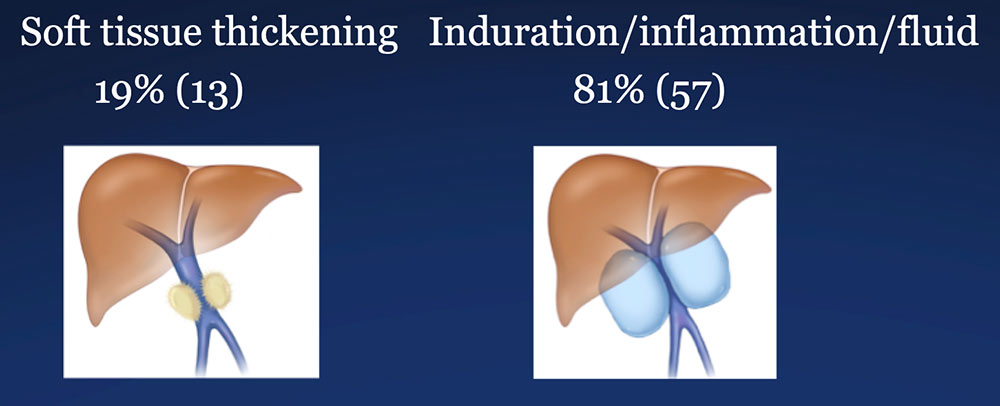 |
Results – Summary The CT appearance of the perivenous space after PVR includes two patterns:  Perivenous soft tissue thickening mimics the appearance of recurrent or residual pancreatic cancer. |
Conclusions Considerable overlap exists between the post-operative appearance of the PV-SMV and surrounding tissues after PVR, and between the appearance of recurrent pancreatic cancer. Post-Op PVR
|
Conclusions
|
Clinical Application Differentiating post-PVR CT findings from recurrent pancreatic cancer The spectrum of CT findings after PD with PVR commonly includes aggressive appearances. Avoid the pitfall of overcalling recurrent local pancreatic cancer.  |
Clinical Application Differentiating post-PVR CT findings from recurrent pancreatic cancer The spectrum of CT findings after PD with PVR commonly includes aggressive appearances. Avoid the pitfall of overcalling recurrent local pancreatic cancer.  Determine surgical details, beyond “Whipple.” Note that a PD might include an unplanned PVR. |
Clinical Application Differentiating post-PVR CT findings from recurrent pancreatic cancer The spectrum of CT findings after PD with PVR commonly includes aggressive appearances. Avoid the pitfall of overcalling recurrent local pancreatic cancer.  Determine surgical details, beyond “Whipple.” Note that a PD might include an unplanned PVR. Establish baseline post-operative pattern. Use coronal images to define post-op PV-SMV configuration. Compare to post-op baseline CT to assess for changes over time. |
References
|
