Review of Current Portal Venous Reconstruction Techniques Performed with Pancreaticoduodenectomy and Correlation with Post-Operative CT Imaging AppearancesReview of Current Portal Venous Reconstruction Techniques Performed with Pancreaticoduodenectomy and Correlation with Post-Operative CT Imaging Appearances The Russell H. Morgan Department of Radiology and Radiological Science The Johns Hopkins Medical Institutions Baltimore, Maryland |
Introduction
|
Introduction
|
The PV-SMV Complex
|
The PV-SMV Complex
|
The PV-SMV Complex 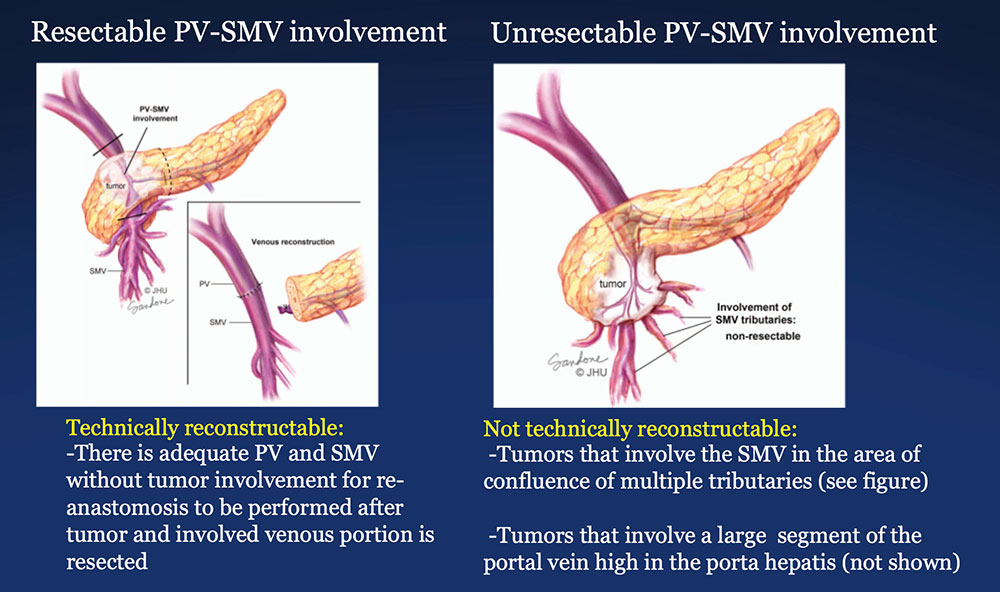 Illustrations by Corinne Sandone © JHU. Used with permission. Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent Progress in Pancreatic Cancer. CA Cancer J Clin. 2013 Sep;63(5):318-48. |
PV-SMV Reconstruction (PVR)
|
PV-SMV Reconstruction (PVR) Reconstruction of a tangential venous resection Venorrhaphy may also be performed for reconstruction of intra-operative venous tears, without tumor involvement of the PV-SMV complex. 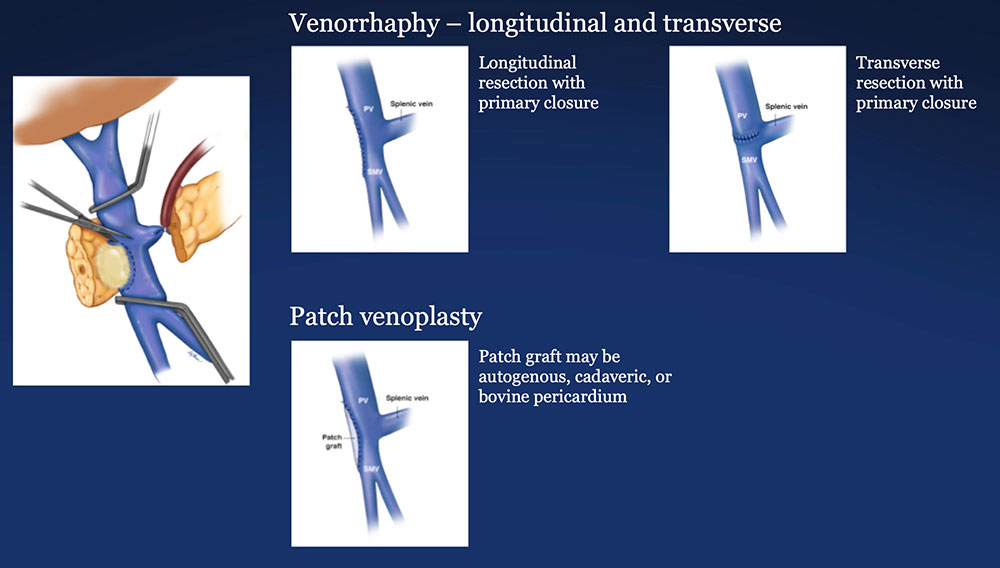 |
PV-SMV Reconstruction (PVR) Reconstruction of segmental venous resection PVR may be performed with or without splenic vein preservation, with preservation preferred if technically possible. Interposition grafts are most commonly autogenous, including IJ and renal vein, but may also be prosthetic. 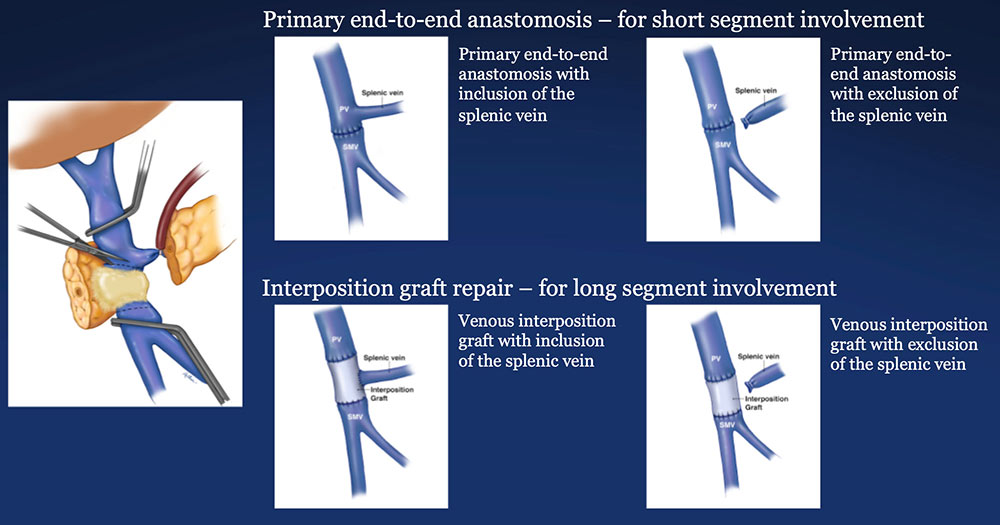 |
CT of PD with PV-SMV Reconstruction All post-PD pancreatic cancer patients at our institution are followed with dual phase pancreatic protocol CT.
|
CT of PD with PV-SMV Reconstruction
|
CT of PD with PV-SMV Reconstruction 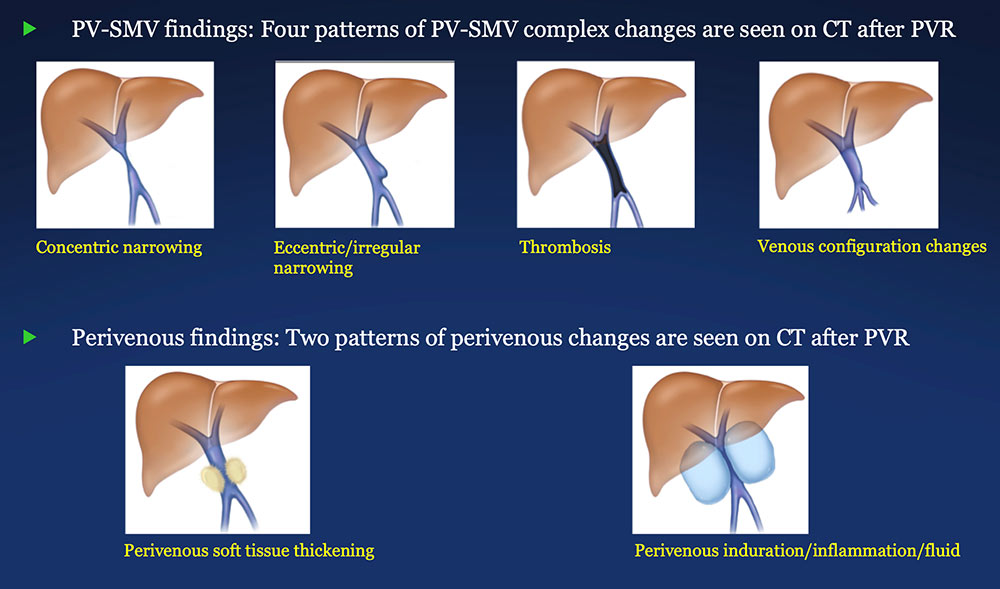 |
PV-SMV: Venous narrowing on CT after PVR 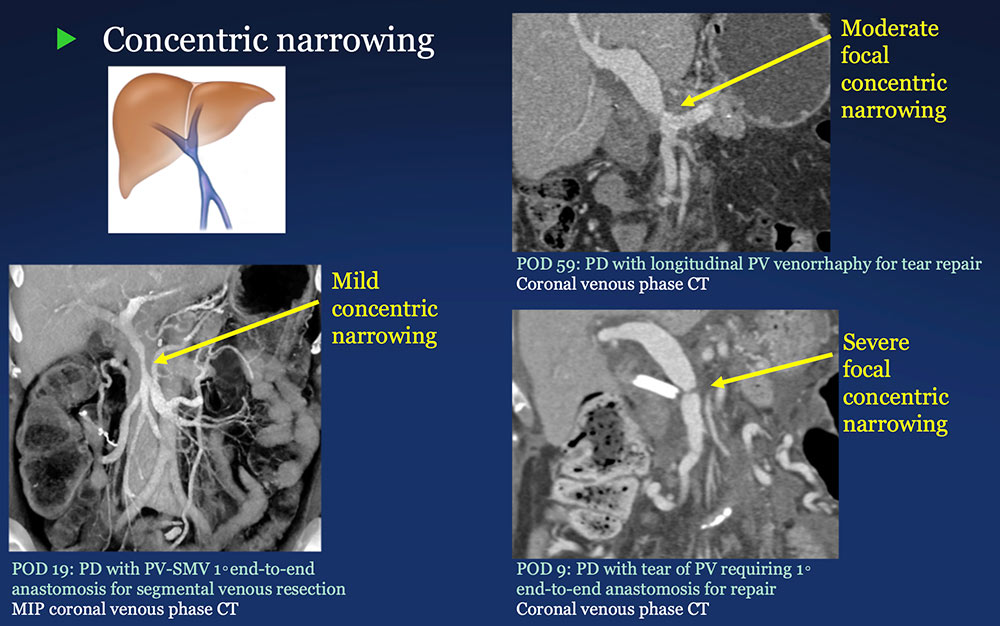 |
PV-SMV: Venous narrowing on CT after PVR 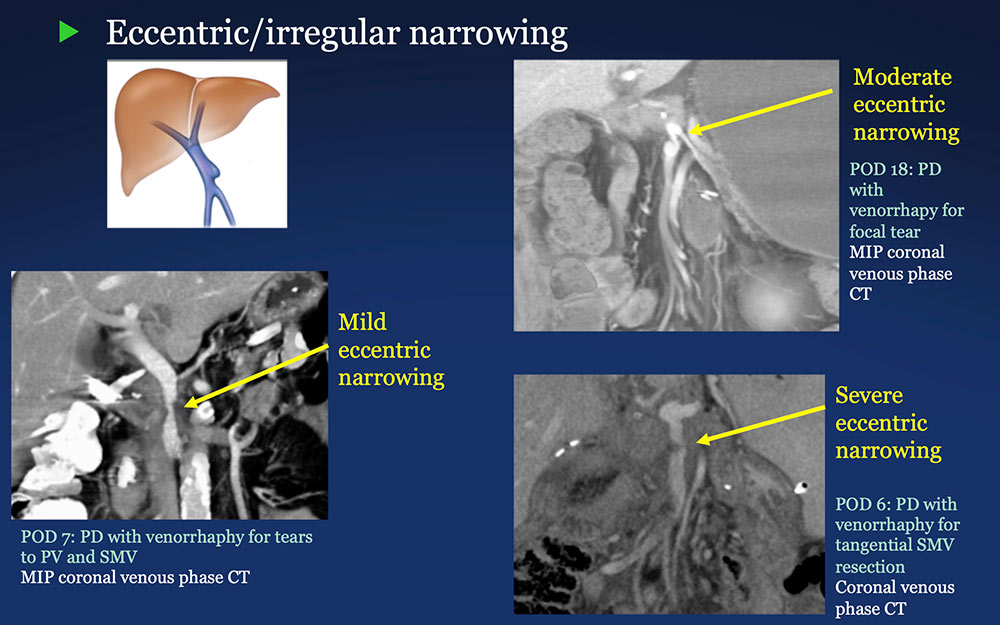 |
PV-SMV: Venous thrombosis on CT after PVR 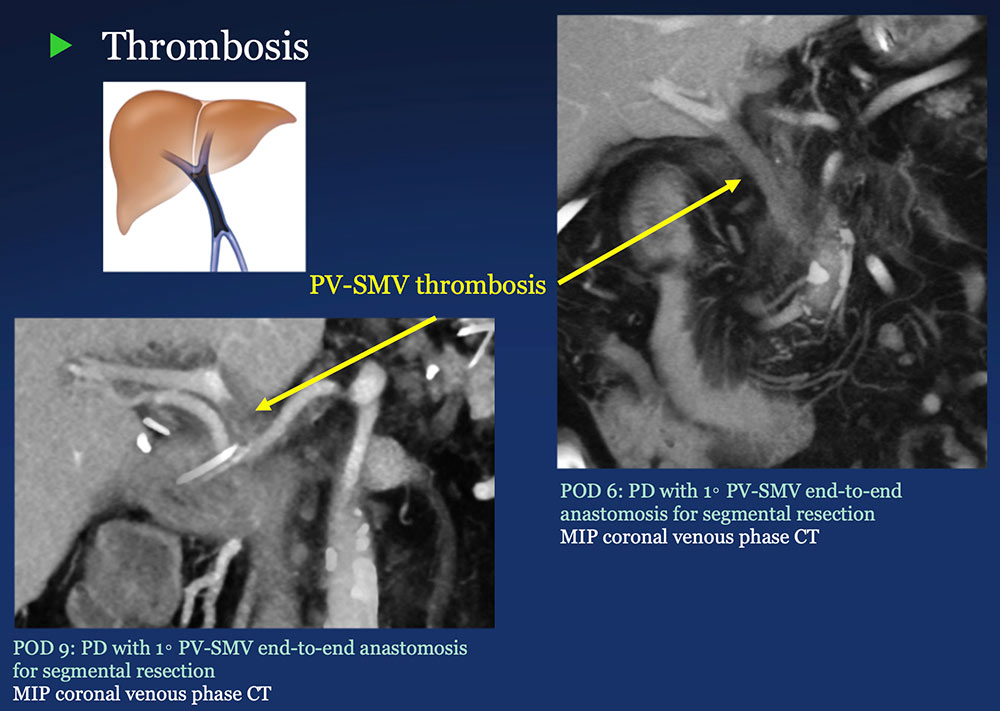 |
PV-SMV: Venous configuration changes on CT after PVR 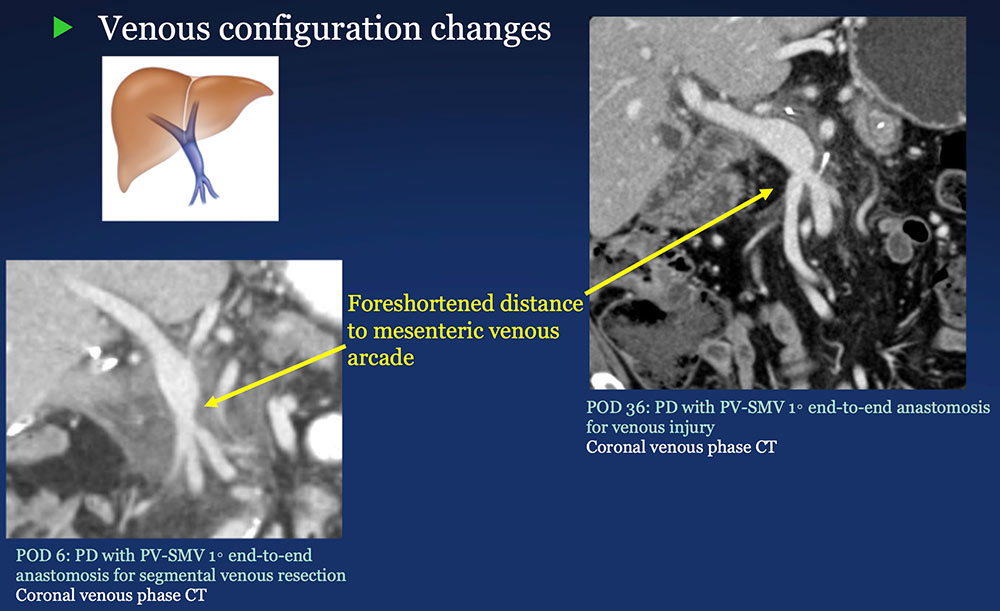 |
PV-SMV: Venous findings on CT after PVR
|
Perivenous space: Perivenous ST thickening on CT after PVR 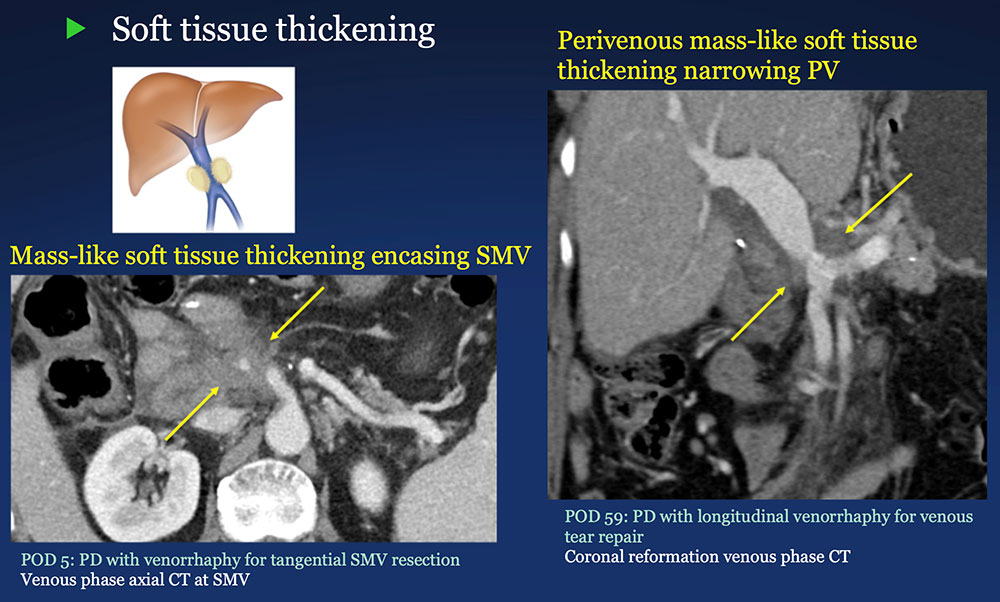 |
Perivenous space: Perivenous ST thickening on CT after PVR 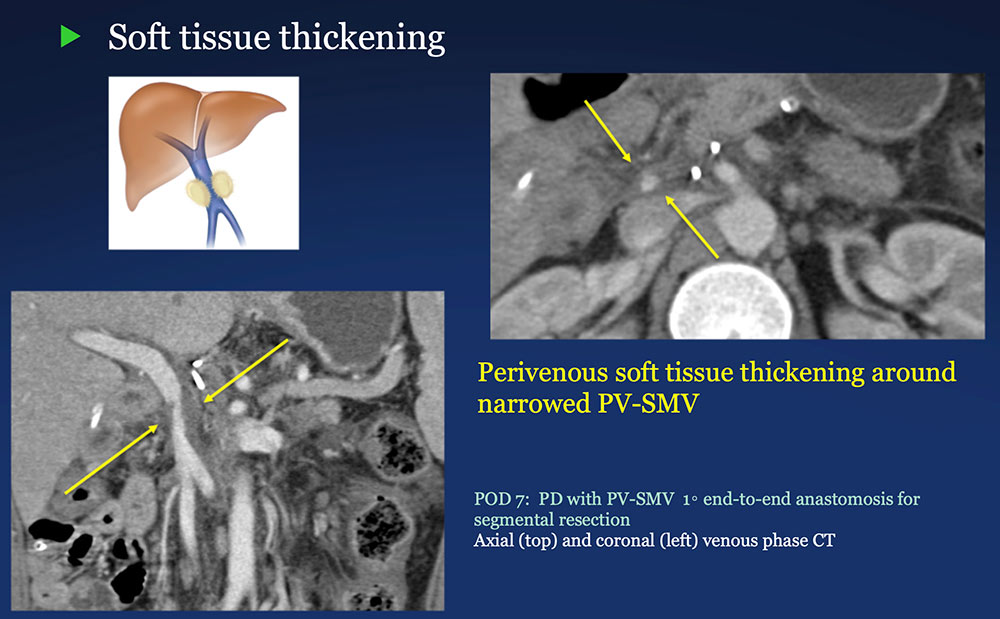 |
Perivenous space: Perivenous ST thickening on CT after PVR 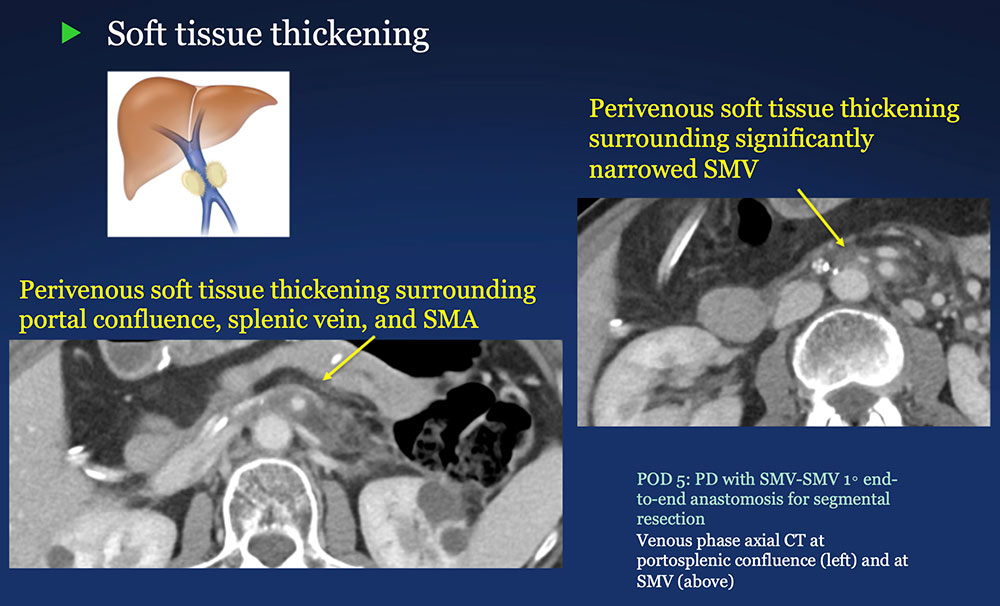 |
Perivenous space: Perivenous ST thickening on CT after PVR 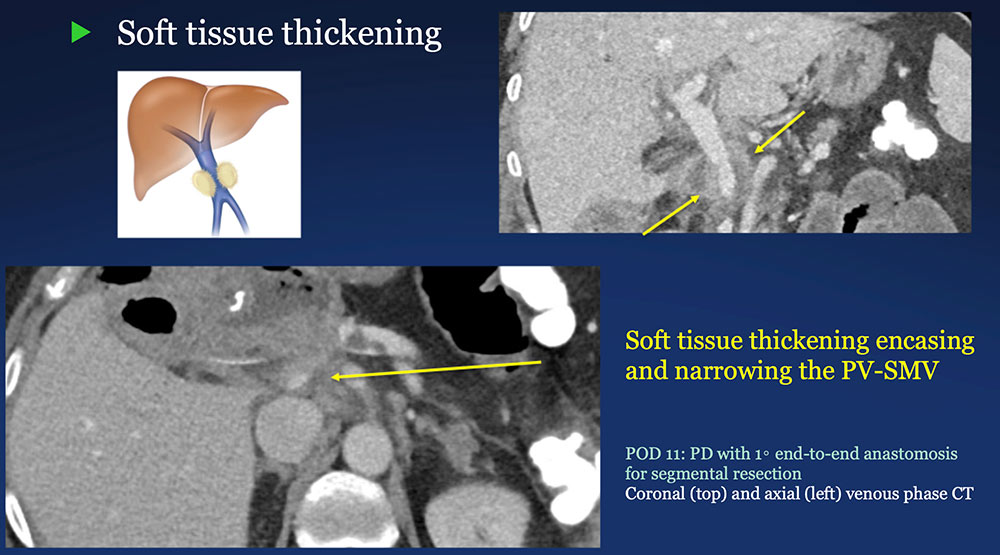 |
Perivenous space: Perivenous ST thickening on CT after PVR 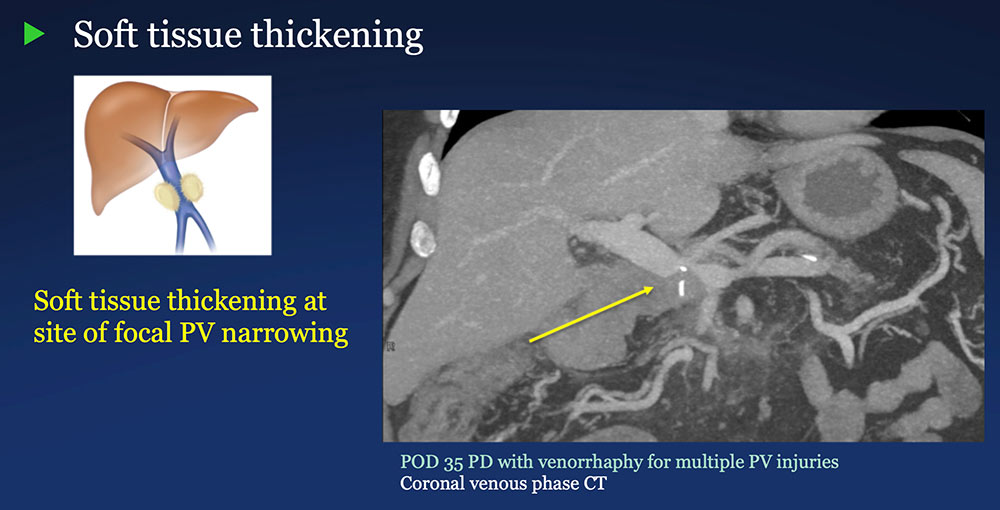 |
Perivenous space: Perivenous ST thickening on CT after PVR 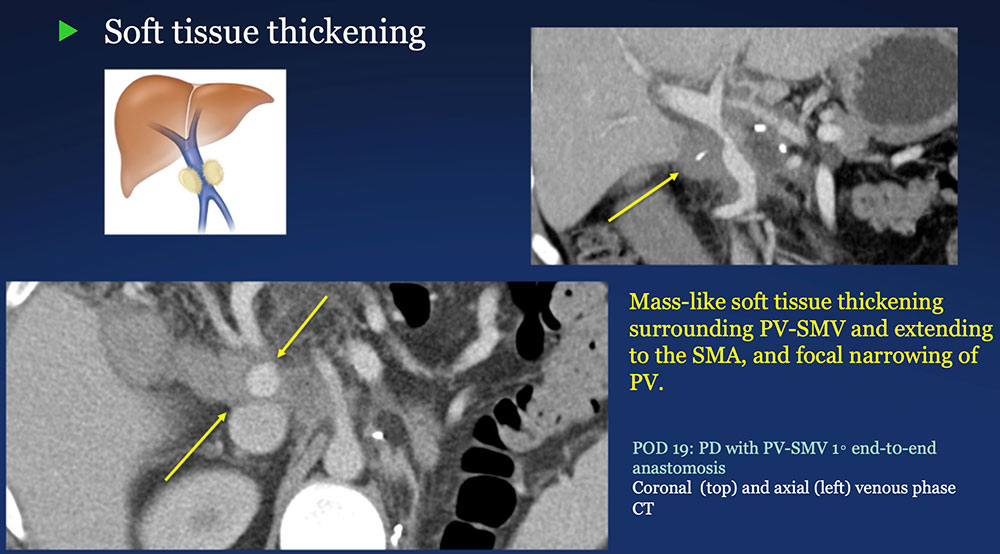 |
Perivenous space: Induration/inflammation/fluid on CT after PVR 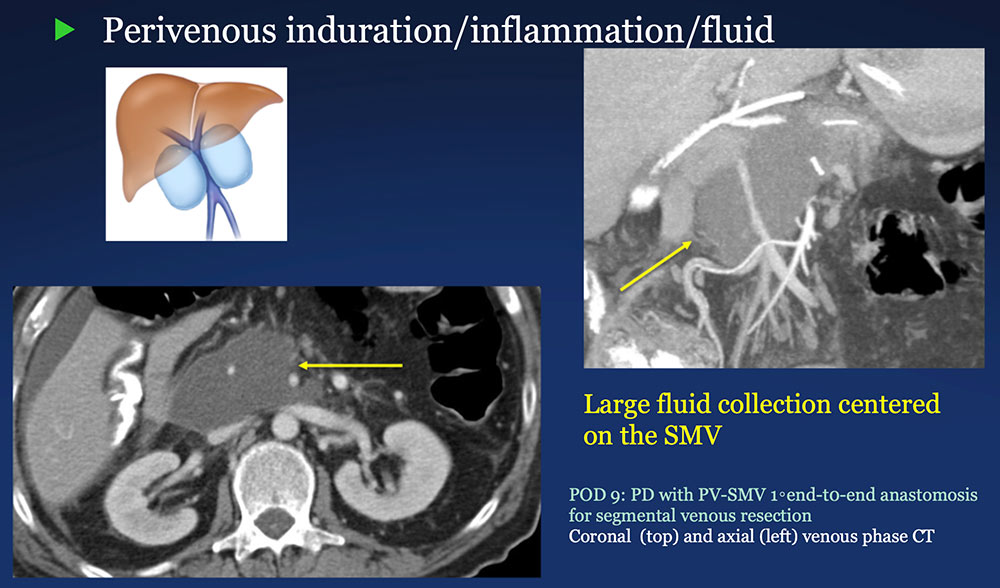 |
Perivenous space: Induration/inflammation/fluid on CT after PVR 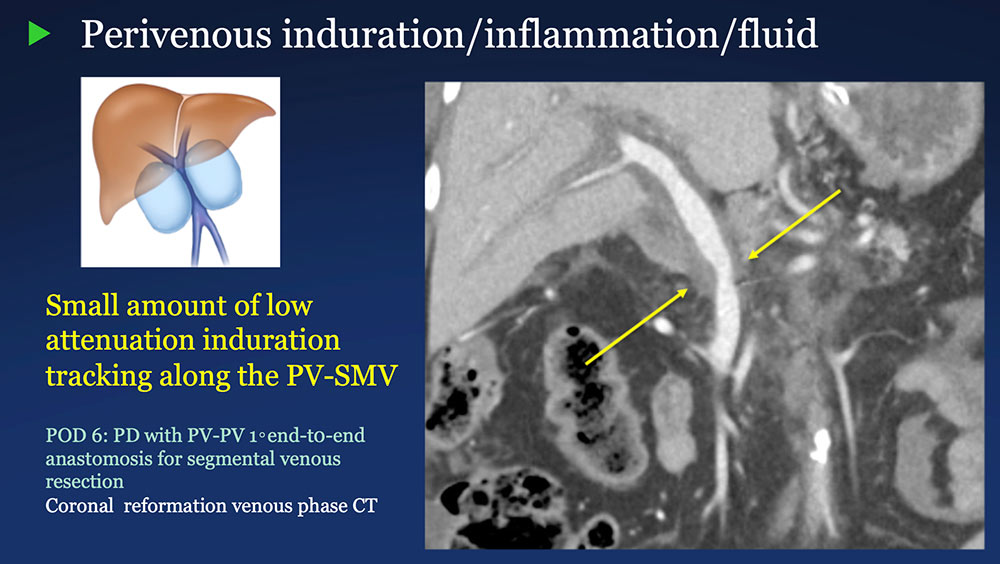 |
Perivenous space: Induration/inflammation/fluid on CT after PVR 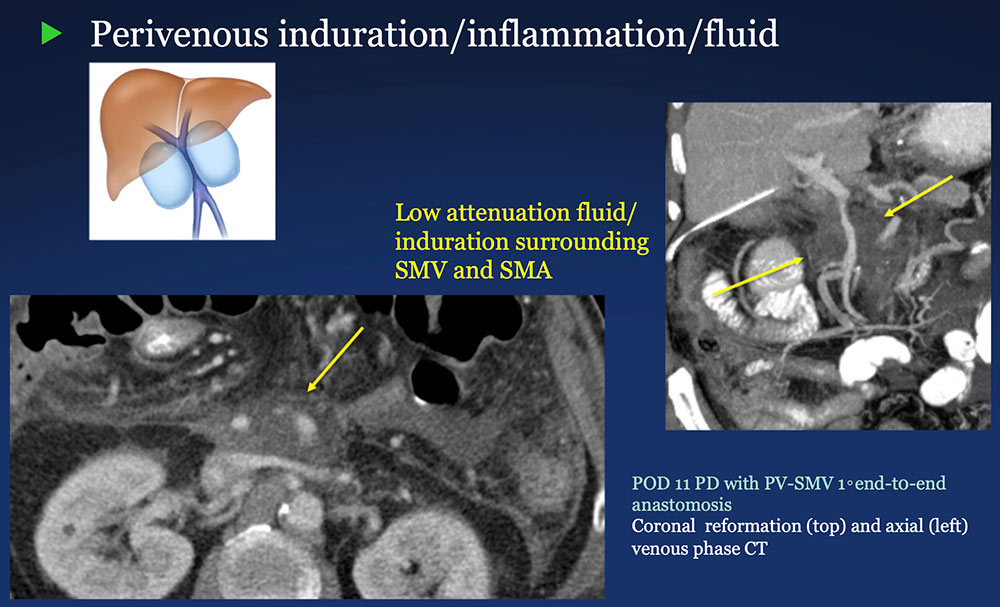 |
Perivenous space: Findings on CT after PVR
|
Post-PVR changes vs. Recurrent pancreatic cancer Post-op PVR
|
Pearls and Pitfalls
|
Pearls and Pitfalls
|
Teaching Points - Review Current venous vascular reconstruction techniques performed in conjunction with pancreaticoduodenectomy for pancreatic cancer Any amount of PV-SMV tumor involvement will be considered for resection as long as it is technically feasible to reconstruct the PV-SMV to maintain blood flow. 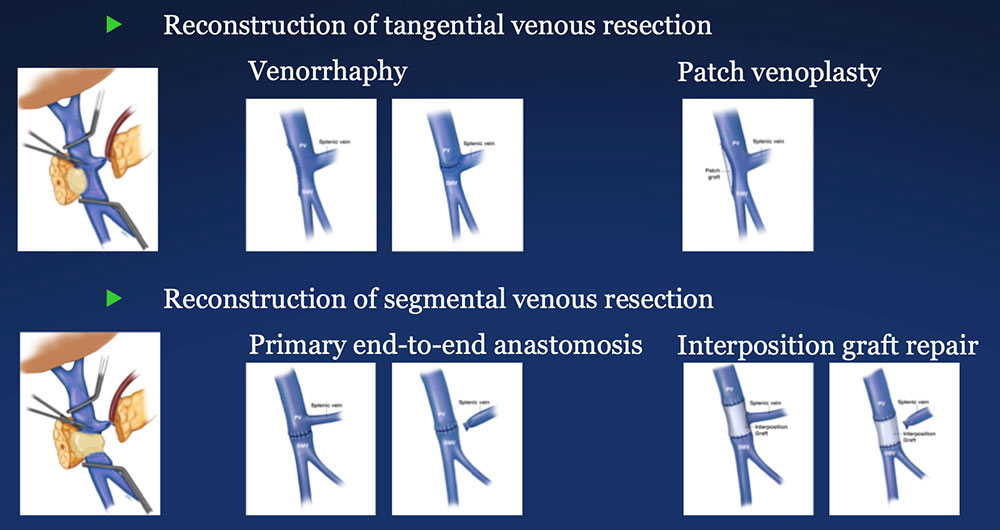 |
Teaching Points - Review Patterns of CT findings following pancreaticoduodenectomy with reconstruction of the PV-SMV complex There are basic patterns of the PV-SMV complex and perivenous space after venous reconstruction, and several of the normal post-operative findings have an aggressive appearance. 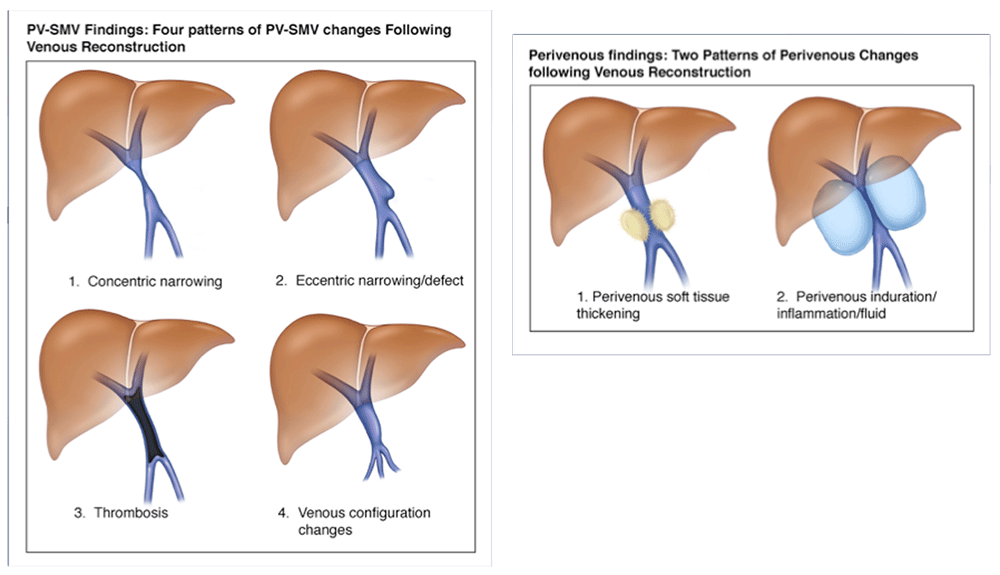 |
Teaching Points - Review Differentiating post-PVR CT findings from recurrent pancreatic cancer Be familiar with normal spectrum of CT patterns post-PVR, including aggressive appearances Avoid the pitfall of overcalling recurrent local pancreatic cancer Determine surgical details, beyond “Whipple” Note that the surgery might include an unplanned PVR. Establish baseline post-op pattern Use coronal images to define post-op PV-SMV configuration. Compare to post surgical baseline to assess for changes over time. 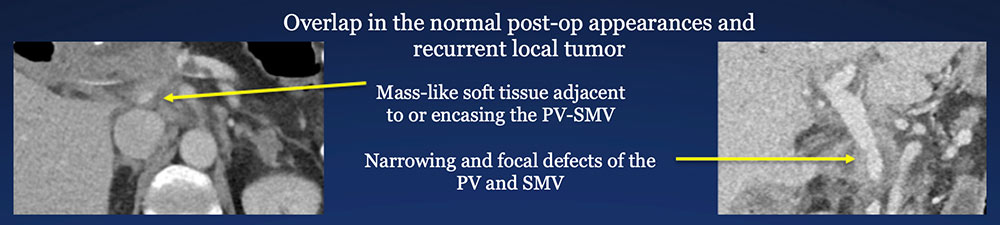 |
References
|
