Imaging Pearls ❯ Chest ❯ COVID-19 Vaccine
|
-- OR -- |
|
- “We present five cases of axillary lymphadenopathy which occurred after COVID-19 vaccination and that mimicked metastasis in oncologic patients. Initial radiologic diagnosis raised concerns for metastasis. However, further investigation revealed that patients received COVID-19 vaccinations in the ipsilateral arm prior to imaging. In two cases, lymph node biopsy confirmed vaccination related reactive lymphadenopathy. Ipsilateral axillary swelling / lymphadenopathy was reported based on symptoms and physical examination in COVID-19 vaccine trials. Knowledge of the potential for COVID-19 vaccine-related ipsilateral adenopathy is necessary to avoid unnecessary biopsy and change in therapy.”
Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients
Can Özütemiz et al.
Radiology (in press) - "In clinical studies, axillary lymphadenopathy was reported on the ipsilateral injection side. Ipsilateral axillary swelling/tenderness was the second most frequently reported local reaction to the Moderna COVID-19 vaccine, occurring in 11.6% and 16.0% of recipients following first and second dose respectively. In the Moderna cohort, clinically detected axillary and supraclavicular lymphadenopathy was reported in 1.1% of study participants within 2-4 days after vaccination, as an unsolicited adverse event. In the Pfizer-BioNTech COVID-19 vaccine trial, the rate of ipsilateral axillary and supraclavicular lymphadenopathy was reported to be 0.3% among vaccine recipients versus <0.1% among placebo group.”
Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients
Can Özütemiz et al.
Radiology (in press) - “Due to widescale vaccination of the majority of the U.S. population, axillary lymphadenopathy due to COVID-19 vaccination is likely to be encountered in oncologic patients. In addition, in this limited series, a triangle of intramuscular inflammation was demonstrated at the injection site on MRI and PET/CT, as also described by Eifer and Eshet , suggesting vaccine-related inflammation. Overall, our findings are important, particularly for cancer patients. Radiologists, oncologists, and internists should be aware of this secondary effect of vaccination to obviate unnecessary changes in management, unnecessary patient emotional stress or biopsy.”
Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients
Can Özütemiz et al.
Radiology (in press) 
- "COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy. A total of 10.2% of patients after the first Moderna vaccine and 14.2% of patients after the second reported palpable adenopathy. Patients receiving the Pfizer vaccination also had increased rates of adenopathy compared to those receiving placebo.”
Mitigating the Impact of Coronavirus Disease (COVID-19) Vaccinations on Patients Undergoing Breast Imaging Examinations: A Pragmatic Approach
Constance D. Lehman, MD, PhD, Leslie R. Lamb, MD, Helen Anne D’Alessandro, MD
AJR (in press) - “Reports of patients with axillary adenopathy identified on breast imaging after coronavirus disease (COVID-19) vaccination are rising. We propose a pragmatic management approach based on clinical presentation, vaccina- tion delivery, and imaging findings. In the settings of screening mammography, screening MRI, and diagnostic imaging work-up of breast symptoms, with no imaging findings beyond unilateral axillary adenopathy ipsilateral to recent (prior six weeks) vaccination, we report the adenopathy as benign with no further imaging indicated if no nodes are palpable six weeks after the last dose. For patients with palpable axillary adenopathy in the setting of ipsilateral recent vaccination, clinical follow-up of the axilla is recommended.”
Mitigating the Impact of Coronavirus Disease (COVID-19) Vaccinations on Patients Undergoing Breast Imaging Examinations: A Pragmatic Approach
Constance D. Lehman, MD, PhD, Leslie R. Lamb, MD, Helen Anne D’Alessandro, MD
AJR (in press) - "For patients with signs or symptoms in the breast or axilla, we also use six weeks to define recent vaccination. For patients with palpable axillary adenopathy absent breast signs or symptoms, clinical follow-up of the axilla is recommended (BI-RADS 2). If clinical concerns persist greater than six weeks after the final vaccine dose, axillary ultrasound is recommended (with mammography if patient is due, or at discretion of the interpreting radiologist). Palpable unilateral adenopathy lasting more than six weeks after vaccination is managed following American College of Radiology BI-RADS recommendations for management of unilateral adenopathy in absence of known infectious or inflammatory cause.”
Mitigating the Impact of Coronavirus Disease (COVID-19) Vaccinations on Patients Undergoing Breast Imaging Examinations: A Pragmatic Approach
Constance D. Lehman, MD, PhD, Leslie R. Lamb, MD, Helen Anne D’Alessandro, MD
AJR (in press) 
- “This study highlights axillary adenopathy ipsilateral to the vaccinated arm with Pfizer-BioNTech or Moderna COVID-19 vaccine as a potential reactive process with which radiologists must be familiar. Recommendation for follow-up imaging may not be warranted. Incorporating the patient’s COVID-19 vaccination history, including vaccination date and laterality, is critical to optimize assessment and management of imaging detected axillary adenopathy in women with otherwise normal breast imaging.”
Coronavirus Disease (COVID-19) Vaccination Associated Axillary Adenopathy: Imaging Findings and Follow-Up Recommendations in 23 Women
Shabnam Mortazavi
AJR (in press) 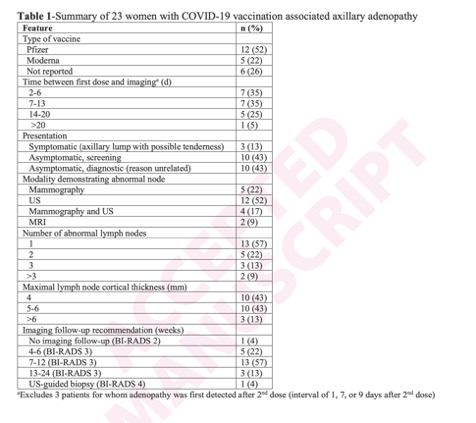
Coronavirus Disease (COVID-19) Vaccination Associated Axillary Adenopathy: Imaging Findings and Follow-Up Recommendations in 23 Women
Shabnam Mortazavi
AJR (in press)
