Imaging Pearls ❯ May 2024
|
-- OR -- |
|
3D and Workflow

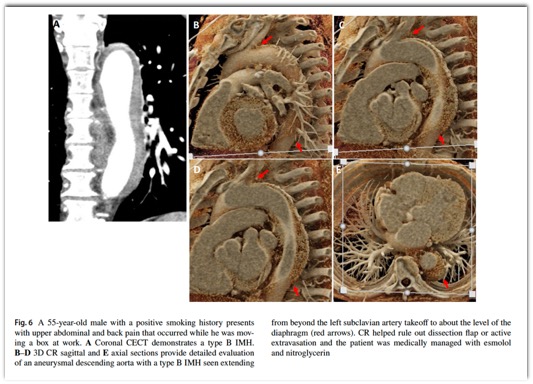
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276.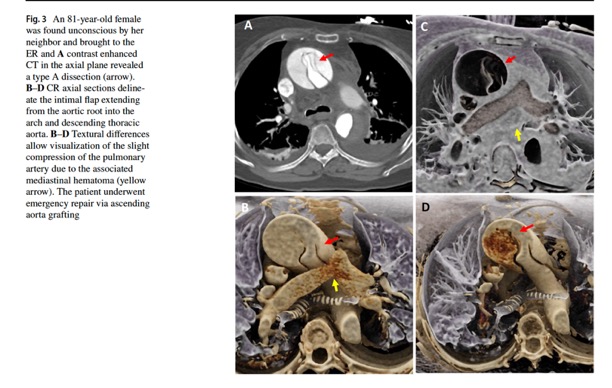
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276.- “Non-traumatic thoracic aorta emergencies are acute conditions associated with substantial morbidity and mortality. In the emergency setting, timely detection of aortic injury through radiological imaging is crucial for prompt treatment planning and favorable patient outcomes. 3D cinematic rendering (CR), a novel rendering algorithm for computed tomography (CT) image processing, allows for life-like visualization of spatial details and contours of highly complex anatomic structures such as the thoracic aorta and its vessels, generating a photorealistic view that not just adds to diagnostic confidence, but is especially useful for non-radiologists, including surgeons and emergency medicine physicians.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “CR involves global illumination and path tracing models whereby numerous light rays from all directions propagate through and interact with the volumetric data to generate a voxel. Complex anatomical relations are better evaluated and enhanced depth and shape perception is achieved as the technique considers a natural lighting environment and its effects (e.g., reflection, diffusion, refraction). Postprocessing windowing and the use of clip planes/masks allow cutting into the volume and isolation of the area/organ of interest.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “As with other 3D post-processing methods, the individual slices are stacked to create a 3D volume, upon which the specialized CR lighting model is applied. This lighting model is unique in that it simulates the propagation of millions of photos through the volume dataset, and thus creates realistic interactions with the various tissue compositions, producing photorealistic images from the dataset. This in turn results in a greater degree of surface detail and shadowing as opposed to traditional VR. Where VR techniques employ a local lighting model, the global illumination model used in CR entails complex calculations for rendering, as both direct and indirect illumination are traced, as well as the effect of diffusion, scatter, and reflections.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “There are some limitations that come with 3D CR. Notably, shadows generated in the images might conceal certain pathologies when viewed from specific angles, necessitating meticulous optimization and assessment from diverse angles in conjunction with the multiplanar reformations. Thus, while an initial learning period to become adept in handling and familiarizing themselves with the CR process is required for radiologists, as demonstrated in our case studies, an experienced radiologist can efficiently execute the rendering process in under 5 min.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “Color mapping of different phases enhances visualization of the key pathology, such as the flow through the false and true lumens in a dissection that can be delineated by high contrast shading. CR rendering emphasizes textural changes attributable to inflammatory processes with realistic shadowing that is otherwise difficult to appreciate. The improved surface detail helps characterize an impending PAU or the nature of outpouchings suspicious for mycotic aneurysms and gives a clearer view of multiple plaques and sites of ulceration that could be otherwise missed.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - Another application of 3D CR is via the black blood cinematic rendering (BBCR) preset.. BBCR is a preset we specifically developed to visualize intraluminal contours and structures of the heart and great vessels, all through adjustments that can be made in under a minute. This is especially useful in the setting of visualizing various zones of thrombi and occlusion, the degree of obstruction, and the subtle irregularities and internal arrangement of the thrombus that can only be appreciated due to enhanced depth perception and shadowing.
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “3D cinematic rendering (CR) represents an important advancement in radiological imaging, particularly in enhancing the visualization of complex anatomical structures and systems such as the thoracic aorta and its vessels. CR provides detailed, photorealistic illustrations crucial for diagnosis and surgical planning as we have seen in several cases. Future research is needed to evaluate CR’s diagnostic accuracy, both prospectively and in head-to-head comparisons with other rendering methods, as well as its role in other domains such as patient education and medical training. CR, therefore, is emerging as a promising, evolving tool for radiologists, surgeons, and the patients they treat.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “Black blood cinematic rendering (BBCR) is a newly described preset for cinematic rendering, which creates photorealistic displays from volumetric data sets with the contrast-enhanced blood pool displayed as dark and transparent. That set of features potentially provides for enhanced visualization of endomyocardial and intraluminal pathology, as well as cardiac devices. The similarity of the images to black-blood magnetic resonance imaging (MRI) may allow for expansion of the evaluation of certain types of pathology into patient populations unable to undergo MRI. In the emergency setting, the rapid acquisition time and reasonable post-processing time make this technique clinically feasible.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “Importantly, in CR, the display color and transparency can be adjusted for each tissue component. This is analogous to window center and level adjustments for standard CT review. Typically, in CR, the contrast-enhanced blood pool is displayed as an opaque light color. Black blood cinematic rendering (BBCR) was a recently described color and transparency preset that displays the contrast-enhanced blood pool as both transparent and dark. That creates images similar to black blood cardiac magnetic resonance imaging (MRI). In our experience, BBCR excels in evaluation of cardiovascular intraluminal structures because of improved detail and delineation from the contrast-opacified blood pool.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “BBCR can be created on any cinematic rendering software by adjusting the trapezoids of the voxel histogram and color Look-Up-Table. Our cardiac BBCR preset was developed at our institution by one of the authors (EKF). It was previously reported and has been published for use . Our preset can be used in SyngoVia 30, 40, 50, and 60. We have not tried this preset on other CR programs, so it is unclear exactly how to replicate it, but theoretically, the underlying concept should apply to all trapezoid-based CR programs. The general concept is to assign 0% opacity to the Hounsfield units of the contrast-enhanced blood pool. Use a narrow-plateau trapezoid to assign color(s) and high opacity to densities less than blood pool to visualize the soft tissues forming the endoluminal walls. Use a wide plateau trapezoid to assign color(s) and high opacity to densities greater than blood pool to visualize calcifications and cardiac implants.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “Further, the images produced by BBCR, although derived from standard CT acquisitions, are also fundamentally different. The pixels on a BBCR image incorporate varying color and transparency based on the proportion of different tissue types that contribute to that pixel. As such, there may be information in BBCR images that cannot otherwise specifically be abstracted from standard reconstructions or reformations. How such images and data might feed into graphical processing unit-based artificial intelligence algorithms is not easily predictable but should be explored.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “With the growing applications of cinematic rendering in cardiovascular imaging, BBCR is valuable for its visualization of intraluminal structures through a dark transparent blood pool. In this expanded experience, we have reviewed our initial experiences with BBCR with cardiac devices, native cardiac valves and coronaries, intracardiac masses, and aortic disease. Given the often-acute presentations of those conditions, many patients may first be evaluated in the emergency setting. Although the creation of any CR images requires a workflow with a standalone workstation and an experienced radiologist, the time to generate BBCR images would be well invested in the relatively rare patient that has an endoluminal cardiac or endovascular condition that needed to be optimally evaluated.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - ”Similar to intracardiac structures, intraluminal vascular structures are well seen on BBCR. Cinematic rendering has been used in the detection of acute aortic injury, and differentiation of blunt aortic injury from ductus diverticulum variant anatomy. Based on the utility of bright blood CR for the aorta, BBCR could add value in the setting of acute aortic syndrome or injury to detect subtle intraluminal irregularities. It can also be used to evaluate larger intraluminal lesions or clots and their relationship to nearby anatomy.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “BBCR captures the cardiac valve anatomy and calcifications. CR has been used in the assessment of aortic valve variant anatomy, and BBCR improves the delineation of the valve cusps from the blood pool. Global valve and outflow tract calcification assessment can be challenging to fully capture on 2D imaging, and routine cinematic rendering displays often obscure the calcifications due to the opaque contrast-enhanced blood pool. BBCR has potential for the assessment of calcifications of the valve leaflets, annulus, and outflow tract in planning for transcatheter valve replacements .”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. 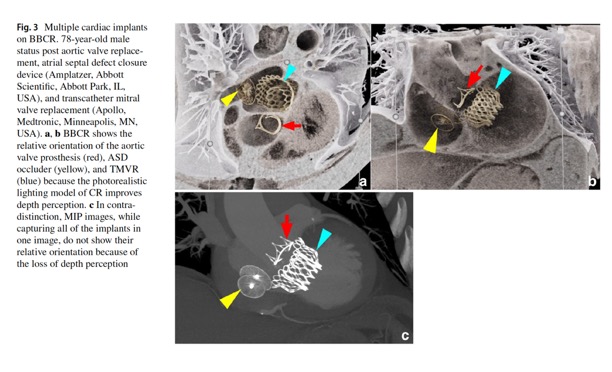
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284.
- “PNETs constitute up to 3% of clinically detected pancreatic tumors. Differentiating PNET from IPAS on CT is challenging as both may appear as well-circumscribed arterial phase enhancing lesions. Cinematic rendering is a 3D postprocessing technology that uses an advanced global lighting mode to generate photorealistic images. The precision with which cinematic rendering simulates intricate interactions of light passing through imaged volumes yields superb surface detail and textural perception.”
Cinematic Rendering for Differentiation of Pancreatic Neuroendocrine Tumor From Intrapancreatic Accessory Spleen.
Ahmed TM, Fishman EK.
AJR Am J Roentgenol. 2024 Mar;222(3):e2430862.
Adrenal
- “The aim of this article is to illustrate the controversial CT hypoperfusion complex in patients with septic shock, characterized by the following imaging features: decreased enhancement of the viscera; increased mucosal enhancement; luminal dilation of the small bowel; mural thickening and fluid-filled loops of the small bowel; the halo sign and flattening of the inferior vena cava; reduced aortic diameter; peripancreatic oedema; abnormal parenchymal perfusion; and other controversial findings that are variably associated with each other and reversible during the early stages. Increasing physicians’ awareness of the significance of these findings could prompt alternative approaches to the early assessment and management of septic shock. In this perspective, CT imaging represents a useful tool for a complete, rapid and detailed diagnosis of clinically suspected septic shock, which can be used to improve patient outcomes.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “The diagnostic accuracy of total-body computed tomography (CT) has been well established for the identification of septic shock, allowing for a rapid and simultaneous study of multiple body areas, generating detailed and panoramic images. The aim of this article is to review the characteristics of septic shock from an imaging perspective, beyond the underlying causes and to highlight how CT can be used to identify a variety of septic shock-related signs that are collectively described as CT hypoperfusion complex. The latter describes a set of widely reported signs and symptoms that are commonly observed during trauma-associated hypovolaemic shock and can be used to identify septic shock. The early recognition, diagnosis, and treatment of septic shock have profound prognostic and therapeutic implications.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “In addition to being used to identify the underlying cause of the septic state, CT is required for the early recognition of shock-associated CT imaging signs, collectively referred to as CT hypoperfusion complex, which can improve patient prognosis and management. The CT hypoperfusion complex is frequently associated with hypotension, which can also present in many no sepsis related clinical conditions, such as trauma-induced hypotensive shock (e.g. severe head or spine injury), cardiac arrest, and diabetic ketoacidosis. The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Di Serafino M, et al.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs. These signs include the decreased enhancement of the viscera, the increased mucosal enhancement and luminal dilation of the small bowel, the mural thickening and identification of fluid-filled loops in the small bowel, the halo sign and flattening of the inferior vena cava (IVC), reduced aortic diameter, peripancreatic oedema and other controversial parenchymal and visceral findings and ascites that can occur in varying combinations and are often and reversible during early stages. The presence of 2 or more vascular, visceral, or parenchymal signs is necessary to establish the presence of CT hypoperfusion complex.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Di Serafino M, et al.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. 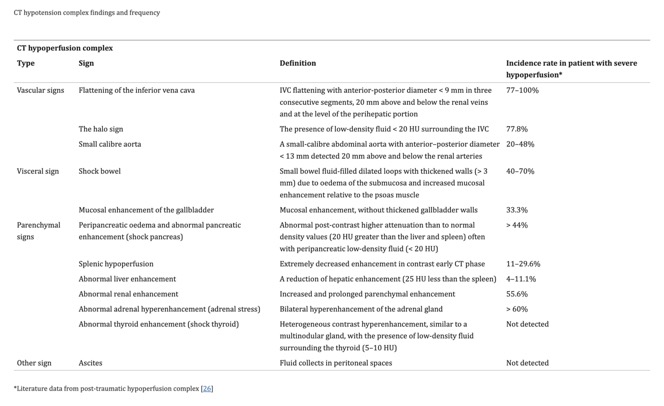
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660.- “Abnormal renal perfusion typically manifests as an increased and prolonged parenchymal enhancement; however, focal and heterogeneous enhancement can also be observed. A fall in systolic pressure causes intense efferent glomerular arteriolar vasoconstriction, which drives glomerular filtration, leading to tubular stasis and the increased resorption of salt and water. Renal parenchymal enhancement is dependent on several factors, including cardiac output and scans timing relative to the injection of contrast agent and, thus, is a non-specific sign. However, kidney enhancement can vary depending on the severity of systemic hypotension. In some cases, unlike hyperenhancement, the decreased enhancement of the renal medulla can be observed in the venous phase, likely due to the impairment of contrast medium outflow from the renal cortex to the medulla, induced by acute renal tubular dysfunction and associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - The bilateral hyperenhancement of the adrenal gland is more common in paediatric cases than in adults and can also present in combination with acute adrenal haemorrhage, which most commonly affects the right side unilaterally, with a homogeneous increase in the size of the gland and the associated suffusion of fat around the adrenal gland. Bilateral adrenal hyperenhancement is the manifestation of adrenergic mechanisms that enhance the blood flow to the vital organs. In the arterial phase, the central zone of the adrenal gland shows less intense enhancement than the peripheral zone or presents a mosaic appearance due to the heterogeneous enhancement of the central zone. In both cases, in the venous phase, the whole adrenal gland is homogenously enhanced. This sign highlights the central role played by the adrenal glands in mediating the sympathetic response to hypotensive shock and is associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. - “In previously published studies, CT hypoperfusion complex has been almost exclusively focused on trauma-induced hypotensive shock and only few studies correlated these signs with prognosis. Some studies have suggested that flattering of IVC, shock bowel, impaired renal enhancement and splenic hypoperfusion are the most suggestive signs of hypoperfusion complex, strongly correlated with a poor prognosis although in a series of trauma-related hypovolemic shock. In contrast, only adrenal hyperenhancement has been correlated with a poor prognosis in septic shock. Despite these contradictory reports, understanding these findings could prompt the development of alternative approaches for the early assessment and management of septic shock in the emergency setting. Therefore, in addition to the application of CT for determining the underlying cause of the septic state, clinicians should be aware and be able to recognize the various CT findings that are suggestive of the hypotensive state. In this perspective, CT imaging represents a useful tool for a complete, rapid, and detailed diagnosis of clinically suspected septic shock, which can be used to improve patient outcomes.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5.
Cardiac
- “The significant number of imaging markers of CV risk, such as CAC score, plaque features, adipose tissue, and radiomics, are being incorporated to traditional clinical risk factors, in order to create predictive models that can increase the performance of current risk score and prognostic models. AI plays an important role in this process, enabling the consideration of clinical and imaging data together while including a larger number of clinical parameters. Several fusion models have been developed to combine multiple resources, and they have been successfully applied to CVD risk and severity assessment, acute CVD detection, and CVD phenotyping.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “Even though CCTA mainly provides anatomic information, thanks to CT-FFR, it can provide functional assessment as well. However, it requires complex computer fluid dynamics computations, which are time-consuming and costly. ML has recently been applied to a CT-FFR calculation (FFRML) as opposed to a computational fluid dynamics (FFRCFD) -based approach in order to shorten execution times. As shown by Tesche and colleagues, FFRML required significantly shorter processing time when compared with FFRCFD, while performing equally in detecting ischemia. Moreover, FFRML closely reproduces FFRCFD calculations, assesses the hemodynamic severity of coronary stenosis, correlating with invasive FFR results, and improves diagnostic accuracy and positive-predictive value of CCTA on a per-vessel and per-patient level.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “CMR, thanks to phase-contrast sequences and the possibility of obtaining specific anatomic planes, has been used to evaluate valves’ anatomy. AI has been applied in this setting to classify and grade valve diseases. Fries and colleagues created a DL model that satisfactorily classified aortic valve malformations from phase-contrast CMR images. They used weak supervision to train a DL model and used it to classify bicuspid aortic valve in unlabeled MR imaging sequences from the UK Biobank. Using health outcome data, they found that the model identified individuals at increased risk of MACE. In addition, ML models have been used to identify different phenotypes of bicuspid valve-associated aortopathy (root, ascending, and arch) and their association with specific clinical findings.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “The significant number of imaging markers of CV risk, such as CAC score, plaque features, adipose tissue, and radiomics, are being incorporated to traditional clinical risk factors, in order to create predictive models that can increase the performance of current risk score and prognostic models. AI plays an important role in this process, enabling the consideration of clinical and imaging data together while including a larger number of clinical parameters. Several fusion models have been developed to combine multiple resources, and they have been successfully applied to CVD risk and severity assessment, acute CVD detection, and CVD phenotyping.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “In summary, AI has been successfully used to perform time-consuming tasks, such as segmentation and postprocessing, optimization of data acquisition and reconstruction, and grading of disease severity. AI has been proven to show an improvement, in terms of time and accuracy, of human work, and therefore, serves as an aid to physicians in better understanding of the patient’s cardiac health. Several AI applications in cardiac imaging demonstrate human-level performance, and it is likely that, in the near future, these applications will be further improved to be integrated into clinical workflow; this will have a great impact on costs, wider usability, and optimization of workflow efficiency.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488
Colon
- Mucocele of the appendix is a descriptive term that refers to dilation of the appendiceal lumen as a result of mucin accumulation and is based on the gross or macroscopic appearance of the appendix. Mucocele formation is most commonly caused by epithelial proliferation, either benign or malignant.
- “Cystic dilatation of the appendix and maximal luminal diameter achieved statistical significance (p < 0.05) for the diagnosis of acute appendicitis with mucocele. Mural calcification achieved statistical significance for one reader (p = 0.0049) and a statistical trend for the other (p < 0.1). A maximal luminal diameter greater than 1.3 cm had a sensitivity of 71.4%, specificity of 94.6%, and overall diagnostic accuracy of 88.2% for the diagnosis of acute appendicitis with mucocele.”
CT Diagnosis of Mucocele of the Appendix in Patients with Acute Appendicitis
Genevieve L. Bennett, et al.
Volume 192, Issue 3 https://doi.org/10.2214/AJR.08.1572 - “Although there is overlap with acute appendicitis without mucocele, CT features suggestive of coexisting mucocele in patients with acute appendicitis include cystic dilatation of the appendix, mural calcification, and a luminal diameter greater than 1.3 cm.”
CT Diagnosis of Mucocele of the Appendix in Patients with Acute Appendicitis
Genevieve L. Bennett, et al.
Volume 192, Issue 3 https://doi.org/10.2214/AJR.08.1572 - “Appendiceal mucocele describes a subset of appendiceal neoplasms including adenoma, low-grade appendiceal mucinous neoplasm (LAMN), high-grade appendiceal mucinous neoplasm (HAMN), and mucinous adenocarcinoma. They are more common in the elderly and up to 50% show rim calcification. An infected mucocele can appear similarly to ruptured appendicitis , but a tubular connection to the cecum is more likely in mucocele than in ruptured appendicitis.”
Review of appendicitis: routine, complicated, and mimics
Joshua C. Hunsaker et al
Emergency Radiology (2023) 30:107–117 - “Significant variation exists in treatment patterns for LAMNs, particularly prior to referral to a high-volume center. Patients frequently underwent colectomy without apparent oncologic benefit. In the current era of high-quality cross sectional imaging, routine use of DL has low yield and is not recommended. Recurrence in this population is rare, and low-intensity surveillance can be offered. Overall prognosis is excellent, even with peritoneal disease.”
Treatment Variation and Long-Term Outcomes of Low-Grade Appendiceal Neoplasms.
White MG, et al.
Ann Surg Oncol. 2023 Dec;30(13):8138-8143. - “Appendicitis is one of the most common sources of abdominal pain in the emergency setting and is generally considered a straightforward diagnosis. However, atypical appearances, non-visualization, and inconclusive features can make these cases more complicated. The objectives of this article are to review the differential diagnoses for right lower quadrant pain, discuss the imaging characteristics of simple appendicitis on computed tomography (CT), and provide guidance for equivocal cases, complicated appendicitis, and appendicitis mimics. This review will also discuss the identification and management of neoplasms of the appendix.”
Review of appendicitis: routine, complicated, and mimics
Joshua C. Hunsaker et al
Emergency Radiology (2023) 30:107–117 - “Dilation of the appendiceal tip with or without calcifications is suspicious for mucocele. Focal dilation of the appendix > 15 mm is 87% specific for mucocele compared to 57% with diffuse dilation. Tip appendicitis could have a similar presentation and findings but should not have any calcifications. Rupture of an appendiceal mucocele results in seeding of the abdomen and may cause pseudomyxoma peritonei. If a patient presents with an appendix > 15 mm with or without calcifications, the report should state concern for appendiceal mucocele with risk for pseudomyxoma if it ruptures.”
Review of appendicitis: routine, complicated, and mimics
Joshua C. Hunsaker et al
Emergency Radiology (2023) 30:107–117 - “The diagnosis of appendicitis is not always straightforward. Presentation may be atypical, and there are a number of pathologies that mimic or complicate the diagnosis. It is important to systematically approach each imaging study with a broad differential and a basic understanding of the overall medical history. Despite the complexity and broad differential, appendicitis accounts for about half of all RLQ inflammation. A systematic approach and search for secondary signs will help the radiologist to differentiate an equivocal appendix from true appendicitis.”
Review of appendicitis: routine, complicated, and mimics
Joshua C. Hunsaker et al
Emergency Radiology (2023) 30:107–117 - “The appendiceal wall thickening reflects mural inflammation of the appendix and represents early change in course of disease which may help pick up cases with early appendicitis. A previous study had showed modest sensitivity (51.9–66.7%) and high specificity (85.2–87.5%) of CT reassessment in diagnosis of appendicitis in patients with equivocal CT findings. However, in their study, the appendiceal wall thickening was subjectively determined by comparing to the normal bowels. This subjective measurement is easy and simple to perform, but there could be potential error in cases with collapsed bowel loops. Kim et al. showed that the appendiceal wall enhancement, intraluminal air in appendix lumen, coexistent inflammatory lesion and appendiceal wall thickening can differentiate appendicitis in patients with equivocal fndings19. Park et al. reported that 78% of the patients with appendicitis had appendiceal wall thickness of≥ 2 mm as compared to 67% of the patients without appendicitis, although this observation did not reach statistical significance.”
Appendiceal wall thickness and Alvarado score are predictive of acute appendicitis in the patients with equivocal computed tomography findings
Massupa Krisem et al.
Scientific Reports | (2023) 13:998 - “A dedicated search for five specific CT findings allowed an overall sensitivity of 94.9% for perforated appendicitis. Among findings with 100% specificity, a focal defect in the enhancing appendiceal wall achieved the highest sensitivity.”
Differentiation of Perforated from Nonperforated Appendicitis at CT
Mindy M. Horrow et al.
Radiology 2003; 227:46–51 - “These results demonstrate poor sensitivity (69.2%) for the diagnosis of perforated appendicitis when using only the three classic CT findings: abscess, extraluminal air, or extraluminal appendicolith. Although inclusion of phlegmon increases sensitivity to 94.9%, it decreases specificity. Alternatively, inclusion of a defect in the enhancing wall yielded 96.4% sensitivity while preserving 100% specificity. For CT examinations performed without intravenous contrast medium or with suboptimal wall enhancement, however, inclusion of phlegmon still improves sensitivity with only a slight decrease in specificity. Considering the five specific findings separately is not clinically useful since the sensitivities of the individual findings are small, ranging from 20.5% for an extraluminal appendicolith to 64.3% for a defect in the enhancing wall.”
Differentiation of Perforated from Nonperforated Appendicitis at CT
Mindy M. Horrow et al.
Radiology 2003; 227:46–51 - “In conclusion, we found that a dedicated search for five specific CT findings— extraluminal air, extraluminal appendicolith, abscess, phlegmon, and a defect in the enhancing appendiceal wall—allows excellent sensitivity (94.9%) and specificity (94.5%) for the diagnosis of perforated appendicitis when evaluated in a group of patients with known appendicitis. A defect in the enhancing appendiceal wall had the highest sensitivity (64.3%) of any individual finding.”
Differentiation of Perforated from Nonperforated Appendicitis at CT
Mindy M. Horrow et al.
Radiology 2003; 227:46–51 - “CT findings of appendicolith, mass effect, and a dilated appendix greater than 13mmare associated with higher risk of treatment failure (≈40%) of an antibiotics-first approach. Therefore, surgical management should be recommended in patients with CT findings of appendicolith, mass effect, or a dilated appendix who are fit for surgery, defined as having relatively low risk of adverse outcomes or postoperative mortality and morbidity. In patients without high-risk CT findings, either appendectomy or antibiotics can be considered as first-line therapy. In unfit patients without these high-risk CT findings, the antibiotics-first approach is recommended, and surgery may be considered if antibiotic treatment fails. In unfit patients with high-risk CT findings, perioperative risk assessment as well as patient preferences should be considered.”
Diagnosis and Management of Acute Appendicitis in Adults: A Review
Dimitrios Moris, MD, MSc, PhD; Erik Karl Paulson, MD; Theodore N. Pappas
JAMA. 2021;326(22):2299-2311 - Acute appendicitis is most frequent during the second and third decades of life, whereas children 9 years or younger have the lowest incidence. Appendicitis is more common among men (male female ratio, 1.4:1), who have a lifetime incidence of 8.6% compared with 6.7% for women. People with a higher income ($44 691 vs $30 027) and education (college-educated vs non– college-educated patients) have a lower incidence of acute appendicitis. The incidence of perforated appendicitis has been increasing despite a decline in the overall incidence of acute appendicitis. Men are more likely to have perforated appendicitis than women (31 vs 25 per 100 000 person-years).
Diagnosis and Management of Acute Appendicitis in Adults: A Review
Dimitrios Moris, MD, MSc, PhD; Erik Karl Paulson, MD; Theodore N. Pappas
JAMA. 2021;326(22):2299-2311 - In addition to the high diagnostic accuracy, CT is a cost-effective tool in guiding management because its application in patients with suspected appendicitis leads to fewer negative appendectomies (defined as operations performed for suspected appendicitis in which the appendix is found to be normal on histologic evaluation), avoidance of unnecessary hospital admissions, and prompt identification of alterative disease processes A limitation of CT is exposure to ionizing radiation. Thus, discussion between physician and patient should take place about the risks and benefits of CT after taking into consideration the individual patient characteristics including age, potential for alternative diagnoses, and pregnancy. However, improvements in imaging technology over the last decade have reduced the radiation dose per scan, without decreasing diagnostic accuracy.
Diagnosis and Management of Acute Appendicitis in Adults: A Review
Dimitrios Moris, MD, MSc, PhD; Erik Karl Paulson, MD; Theodore N. Pappas
JAMA. 2021;326(22):2299-2311 - “Appendicoliths are incidentally discovered by CT in approximately 4% of asymptomatic patients. However, approximately 40% of patients with acute appendicitis have appendicoliths identified by CT. Ranieri et al showed that in 248 patients with acute appendicitis, the presence of appendicoliths was associated with more extensive or severe inflammation and an increased (38.7% vs 4.4%) risk of perforation (P < .05 for both).”
Diagnosis and Management of Acute Appendicitis in Adults: A Review
Dimitrios Moris, MD, MSc, PhD; Erik Karl Paulson, MD; Theodore N. Pappas
JAMA. 2021;326(22):2299-2311 - Appendiceal neoplasms are uncommon tumors of the gastrointestinal tract that may manifest with symptoms of appendicitis, right lower quadrant pain, or palpable mass, leading to imaging or surgical intervention. The majority of appendiceal masses consist of primary epithelial neoplasms and neuroendocrine tumors (NETs). Epithelial neoplasms—mucinous and nonmucinous types—are more often detected at imaging than NETs due to their larger size and propensity for peritoneal spread and metastatic disease. Epithelial mucinous neoplasms are defined by the presence of mucin, detected at radiologic and pathologic examination. A mucocele or pseudomyxoma peritonei from epithelial mucinous tumors are the two most common cross-sectional imaging findings of appendiceal mucinous neoplasms. Nonmucinous epithelial tumors are less common and manifest as masses similar to colonic-type malignancies.
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “Primary and secondary neoplasms of the appendix are rare tumors found in approximately 1% of appendectomy specimens. Epidemiologic studies have shown increased incidence and decreased age at diagnosis of appendiceal tumors, likely due to better detection through noninvasive imaging and colonoscopy. The most common appendiceal tumors are epithelial neoplasms and neuroendocrine tumors (NETs). Other tumors are rarely encountered and include lymphoma, metastases, neuroectodermal and nerve sheath tumors, mesenchymal tumors, and Kaposi sarcoma.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “Imaging manifestations of PMP include mucinous ascites, peritoneal soft-tissue implants, omental caking, and involvement of the gastrointestinal tract and ovaries. Cross-sectional imaging demonstrates loculated peritoneal collections that can progress to large-volume ascites. The solid abdominal organs develop a scalloped appearance due to mass effect by tumoral implants, and parenchymal invasion can occur at the sites of serosal involvement. Linear or punctate calcifications of the mucinous deposits may be identified. The hollow viscera are displaced and distorted, and small-bowel obstruction due to tumor deposits is a common complication in advanced disease.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “Characteristic imaging features of nonmucinous epithelial neoplasms include a focal soft-tissue mass or subtle soft-tissue infiltration of the entire appendix, without mucocele formation. Periappendiceal fat stranding is common and in the setting of diffuse mural thickening may be due to extension of the primary tumor and/or superimposed appendicitis. Cross sectional evaluation demonstrates direct invasion of the adjacent organs, regional and distant lymphadenopathy, and metastatic disease. The most common sites of spread include the peritoneum, ovaries, liver, and lung.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “NETs of the appendix are often difficult to visualize at cross-sectional imaging due to their small size, with a mean diameter of less than 1 cm. Seventy-five percent of NETs involve the distal appendix, while 25% occur at the base and may cause appendicitis. The lesions typically manifest as small submucosal masses or nodular wall thickening. NETs typically enhance avidly and may contain calcifications, mimicking appendicoliths.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “Similar to other gastrointestinal lymphomas, primary appendiceal lymphoma manifests as diffuse wall thickening and enlargement, with preservation of the vermiform shape. Aneurysmal dilatation of the lumen is characteristic but not always present. Regional lymphadenopathy and bowel involvement are seen when the appendix is secondarily involved.” "
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083 - “CT with or without intravenous and oral contrast material is used at many institutions as the initial imaging modality for evaluation of right lower quadrant or diffuse abdominal pain, which may lead to detection of appendiceal tumors . Using diagnostic criteria of dilatation of appendiceal diameter of over 15 mm or morphologic changes of appendiceal cystic dilatation or a soft-tissue mass, the sensitivity of CT for detecting appendiceal tumors is 95% in patients with symptoms of appendicitis. Use of oral and intravenous contrast material aids in identification and staging of appendiceal tumors by delineating the bowel, primary tumor, and metastatic deposits.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 - “At CT, a mucocele manifests as a dilated appendix filled with homogeneous low-attenuation material. Mural nodularity and irregular wall thickening are features that have been associated with malignant mucoceles due to adenocarcinoma. The shape, attenuation of internal content, maximal wall thickness, and presence of internal septa, wall calcifications, periappendiceal fat stranding, or intraperitoneal free fluid are not helpful in differentiating malignant from benign mucoceles.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 - “PMP is characterized by a redistribution phenomenon, which determines the pattern of peritoneal spread and the imaging appearance. The mucus and cells follow the normal flow of peritoneal fluid. Deposits accumulate in the pelvis and paracolic gutters, and on the omentum and liver capsule. Initially, PMP tends to spare the mobile small intestine, although mesenteric involvement and polypoid tumor deposits on bowel loops may be present in advanced disease. The typical manifestation of PMP is slow but progressive intraperitoneal growth, without distant metastases.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 - “In patients with mucinous neoplasms and peritoneal spread of disease, a combined approach consisting of laparoscopy, CRS, and HIPEC is considered the standard of care. The goal of CRS is complete resection of all visible tumor, defined as removal of all tumor deposits greater than 2.5 mm. Laparoscopy plays an important role in management of patients with peritoneal spread. The addition of laparoscopy to preoperative CT, physical examination, and endoscopy improves the completion rates for CRS and HIPEC from 56% to 70% .”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 - “The gastrointestinal tract is the most common site of involvement by extranodal lymphoma; however, primary appendiceal lymphomas are rare. The appendix is most often secondarily involved by contiguous spread from nodal masses or adjacent bowel. The most common clinical presentations are right lower quadrant pain and acute appendicitis. Appendiceal lymphomas are due to non-Hodgkin disease, with mantle cell and diffuse large B-cell histologic types being the most common. Appendiceal involvement is characterized by infiltration of the appendiceal wall by lymphomatous cells.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 - “Imaging findings may overlap with those of acute appendicitis, including fat stranding and regional adenopathy. Features that should alert the radiologist to the presence of an underlying neoplasm are irregular wall thickening of the appendix, enhancing soft-tissue nodularity, infiltration, or a mass. The omentum, peritoneal spaces, bowel, and solid abdominal and pelvic organs should be scrutinized for mucinous or soft-tissue deposits. Metastatic disease to the liver and lung is uncommon. CT is often used for staging and follow-up of appendiceal tumors, although MR imaging is a more optimal modality for evaluation of peritoneal disease. In the setting of PMP, the radiologic PCI guides the surgical approach. CRS and intraperitoneal chemotherapy have improved the prognosis of patients with peritoneal disease.”
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1079–1083 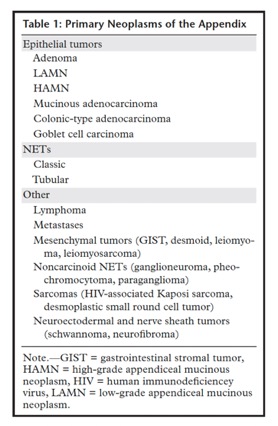
Neoplasms of the Appendix: Pictorial Review with Clinical and Pathologic Correlation
Laura M. Leonards et al.
RadioGraphics 2017; 37:1059–1083
Deep Learning
- “How different is this situation from the developments in medicine where physicians are giving away their knowledge to artificial intelligence (AI) on a voluntary basis and spend hours of valuable research time sharing expert knowledge with AI systems. AI has entered the medical field so rapidly and unobtrusively that it seems as if its interactions with the profession have been accepted without due diligence or in-depth consideration. It is clear that AI applications are being developed with the speed of lightning, and from recent publications it becomes frightfully apparent what we are heading for and not all of this is good. AI may be capable of amazing performance in terms of speed, consistency, and accuracy, but all of its operations are built on knowledge derived from experts in the field. We here follow the example of the kidney pathology field to illustrate the developments, emphasizing that this field is only exemplary of other fields in medicine.”
AI's Threat to the Medical Profession.
Fogo AB, Kronbichler A, Bajema IM.
JAMA. 2024 Feb 13;331(6):471-472. - “This era will show a decrease in intellectual debates among colleagues, a sign of the time that computer scientists have already warned us about. While authors of literature are fighting for regulations to control the usage of AI in art, physicians should contemplate how to take advantage of the potential benefits from AI in medicine without losing control over their profession. With the issue of a landmark Executive Order in the US to ensure that America leads the way in managing the risks of AI and the EU becoming the first continent to set clear rules9 for the use of AI, physicians should realize that keeping AI within boundaries is essential for the survival of their profession and for meaningful progress in diagnosis and understanding of disease mechanisms.”
AI's Threat to the Medical Profession.
Fogo AB, Kronbichler A, Bajema IM.
JAMA. 2024 Feb 13;331(6):471-472. 
- “Even among industry leaders, there is a wide variety in maturity levels, as health systems approach AI from different angles and baselines. This roadmap will be a valuable tool for guiding best practices, investments, and conversations, and aligning progress to help health systems take advantage of sector-wide advancement. The AI Collaborative continues to meet, discuss, and refine the AI Maturity Roadmap, with plans to expand the Business Implementation, Value, Maintenance and Operations, and Information Architecture sections.”
The AI Maturity Roadmap: A Framework for Effective and Sustainable AI in Health Care
Peter Durlach et al.
NEJM AI DOI: 10.1056/AI-S2400177 - “Researchers expect the deficit of primary care physicians to reach as much as 55,200 by 2033, and a shortage of non-primary specialty physicians of up to 86,700. This is due to a combination of the factors above, and intensified by the fact that more than two-fifths of currently active physicians will reach the standard retirement age within the next decade. With so many experiencing burnout, it’s likely that these physicians will seek to accelerate their retirement, rather than extend their careers.”
The AI Maturity Roadmap: A Framework for Effective and Sustainable AI in Health Care
Peter Durlach et al.
NEJM AI DOI: 10.1056/AI-S2400177 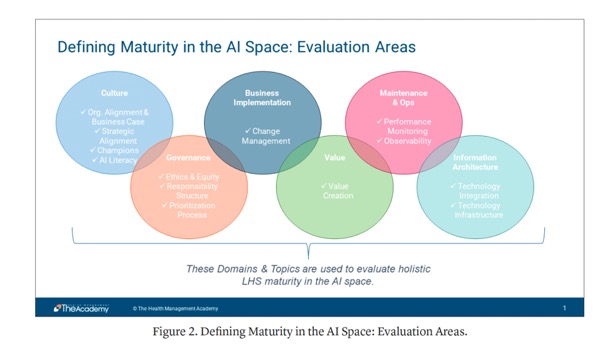
The AI Maturity Roadmap: A Framework for Effective and Sustainable AI in Health Care
Peter Durlach et al.
NEJM AI DOI: 10.1056/AI-S2400177- ”Deployed strategically, AI has a key role to play in the future of health care, supporting everything from individual diagnoses to broad organizational strategy. As it continues to evolve, the AI Maturity Roadmap can function as a framework for all health systems on this journey.”
The AI Maturity Roadmap: A Framework for Effective and Sustainable AI in Health Care
Peter Durlach et al.
NEJM AI DOI: 10.1056/AI-S2400177
- The early detection of pancreatic cancer is a critical factor in improving patient outcomes, as it is often diagnosed at an advanced stage when treatment options are limited. AI has the potential to aid in the early detection of pancreatic cancer by analyzing medical data and identifying patterns that may indicate the presence of the disease. Deep learning techniques can be trained on large datasets to accurately identify early stage pancreatic cancer based on characteristic imaging features or use morphology features to build segmentation frameworks for the pancreas. AI algorithms can integrate various patient data, such as age, family history, lifestyle factors, and medical history, to detect an individual’s developing pancreatic cancer early. AI can also analyze a patient’s electronic health records, including medical history, laboratory results, and diagnostic reports, to identify potential indicators of pancreatic cancer. By processing and interpreting vast amounts of data, AI algorithms can detect subtle patterns and abnormalities that may go unnoticed by clinicians.
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; 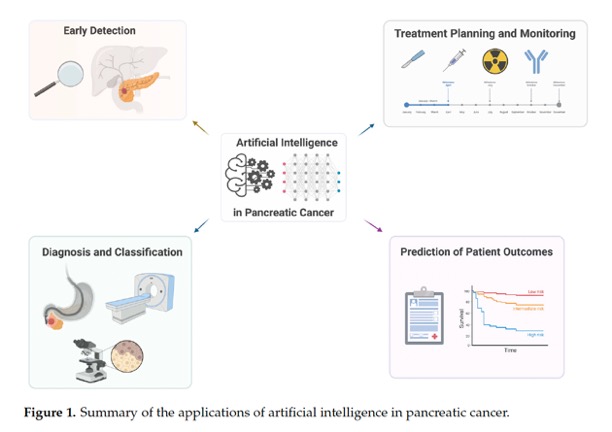
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051;- “The lack of large, centralized datasets that can be used to build and test algorithms poses a barrier to developing comprehensive models. Currently, there is only one major effort in addressing this through the NIH-NCI-sponsored EDRN project for pancreatic cancer. Studies that have used smaller available datasets have not accounted for suboptimal image quality and factors that make images unsuitable for AI, such as posttreatment status and the presence of biliary stents. These gaps in the quality of the data used to develop models may result in errors and biases that limit their applications in clinical medicine.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “AI can serve as a powerful tool in the advancement of pancreatic cancer diagnosis, management, and prognosis, particularly in identifying tumors earlier in disease progression. Despite the many applications and advantages of AI in pancreatic cancer, multiple limitations pose challenges that must be addressed as the field grows. One is the lack of a standardized approach to treatment and diagnosis. Other challenges include a lack of robust and high-quality data, transparency and reproducibility of findings, and ethical considerations, including biases in algorithms.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - Furthermore, AI algorithms have been previously referred to as “Black boxes” due to their lack of transparency and interpretability. The opacity of the code used to build AI models and the hidden level of complexity make it difficult to reproduce results in an independent manner. General descriptions of the code used to build models do not provide enough information to reproduce most findings. The lack of easy interpretation of these AI models and prospective studies assessing AI-based tools has increased the hesitancy of adaptation into clinical practice. Without transparency and interpretation, clinicians are not able to critically interrogate the output of these models, putting an incredible amount of faith in the accuracy of the model. Improving reproducibility and interpretability will be crucial challenges to overcome prior to the clinical adaptation of AI models in pancreatic cancer.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Lastly, a few ethical concerns should be considered when discussing the implementation of AI in pancreatic cancer. Datasets used to build models tend to lack data from underrepresented groups such as women and minorities, leading to biased models that may not be applicable to the diverse patient population seen clinically. Implementing these skewed algorithms can increase disparities in health outcomes between groups rather than improving outcomes, particularly because models tend to perform best on data that are most like the data they were trained with. Improving the diversity in patient data used to train models and validating models across various populations could mitigate this challenge and provide models that are more generalizable to a heterogeneous patient population. Additionally, the creation and use of large datasets needed to create AI models pose the challenging questions of data ownership and patient privacy, particularly in reference to medical imaging. At the same time, the integration of AI systems in medical practices raises questions about the security and confidentiality of sensitive patient data. Ensuring robust data protection mechanisms is imperative to prevent unauthorized access and potential misuse of personal health information.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Pancreatic cancer is a complicated disease with molecular heterogeneity. Integrating multi-omics data, such as genomes, transcriptomics, proteomics, and metabolomics, can offer a complete picture of the disease pathology. Future research should concentrate on building artificial intelligence algorithms capable of assessing and combining these disparate datasets in order to uncover strong molecular signatures, biomarkers, and therapeutic targets for pancreatic cancer.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Current guidance for pancreatic cancer surveillance is restricted to high-risk individuals (HRIs) who have germline mutations that predispose to a lifetime increased risk of pancreatic cancer or a strong family history of pancreatic cancer. When a pancreatic cyst is detected incidentally by abdominal imaging, such patients are often put in the HRI category for surveillance. Cumulatively, HRIs account for only about 20–25% of cases. What about the majority of patients with no risk factors who present at an advanced stage? This is where advances in artificial intelligence (AI) based on mining of electronic health records (EHRs) have started to show some promise. ”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - “ It is important to emphasize that these are retrospective studies and have not yet been evaluated prospectively in a real-world setting. Nonetheless, such research provides a potential roadmap for early detection of pancreatic cancer that extends beyond the current narrow definition of HRIs by enriching the general population with a larger proportion of individuals at “sporadic” risk who are identified through the mining of EHR data (figure). If prospective studies support this approach, this enriched population could then undergo longitudinal surveillance using liquid biopsy tools (circulating tumour DNA, methylation assays, or proteins) that are being deployed in the context of early detection of multiple cancers.”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - ”The contribution of AI does not stop at the initial EHR-based enrichment step since deep learning models are also being developed to improve the resolution of CT and MRI imaging scans for the detection of early, subcentimetric cancers in the pancreas. In addition to changes within the pancreas, these computational algorithms could also identify subtle changes in body composition (eg, attenuation of visceral fat and muscle) that may be missed by clinicians. With such surveillance of a high-risk group, early diagnosis would be enabled, as would the potential for improving outcomes, with treatment including surgical resection followed by emerging immunotherapy options, such as personalised vaccines. Therein lies the opportunity for AI support to help advance diagnosis and care for pancreatic cancer. ”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - “ In a study that used a transformer AI model that incorporated time sequence data of longitudinal EHRs over several years, an aggregate of nearly 28 000 cases of pancreatic cancer were analyzed and compared with 11 million patients who did not develop this disease. The primary dataset was from over 6 million patients in a Danish national registry, and findings were subsequently validated in an additional 3 million patients in the US Veterans Affairs system. The authors were able define a group of people among those aged 50 years and older who had a 30–60 times higher risk than the general population of being diagnosed with pancreatic cancer within the next 12 months. One of the EHR diagnostic codes that the model consistently identified as a feature predictive of incident pancreatic cancer within the next 24 months was diabetes, reinforcing the established link between new-onset diabetes and underlying pancreatic cancer. A second independent study used AI to differentiate the approximately 35 000 patients who developed pancreatic cancer from 1·5 million people who did not. This study identified over 80 features derived from EHRs, laboratory tests, symptoms, medications, and coexisting conditions that defined increased risk. Some of the features within the algorithm are intuitive, such as age or diabetes, whereas others underscore how AI can identify patterns not readily discernible by human assessment (eg, mean corpuscular haemoglobin concentration in red blood cells).”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - “The challenges faced during implementation are to ensure that data used by AI systems are accurate, complete, and interoperable across different healthcare systems. In addition, other challenges include navigating complex regulatory frameworks, gaining acceptance from healthcare professionals who may be skeptical about relying on AI recommendations for patient care, balancing the costs associated with implementing AI solutions against the expected benefits, and demonstrating a clear ROI. These challenges require collaboration among healthcare professionals, technology developers, policymakers, and regulatory bodies to create a supportive and secure environment for the integration of AI in healthcare workflows.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051;
- “The significant number of imaging markers of CV risk, such as CAC score, plaque features, adipose tissue, and radiomics, are being incorporated to traditional clinical risk factors, in order to create predictive models that can increase the performance of current risk score and prognostic models. AI plays an important role in this process, enabling the consideration of clinical and imaging data together while including a larger number of clinical parameters. Several fusion models have been developed to combine multiple resources, and they have been successfully applied to CVD risk and severity assessment, acute CVD detection, and CVD phenotyping.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “Even though CCTA mainly provides anatomic information, thanks to CT-FFR, it can provide functional assessment as well. However, it requires complex computer fluid dynamics computations, which are time-consuming and costly. ML has recently been applied to a CT-FFR calculation (FFRML) as opposed to a computational fluid dynamics (FFRCFD) -based approach in order to shorten execution times. As shown by Tesche and colleagues, FFRML required significantly shorter processing time when compared with FFRCFD, while performing equally in detecting ischemia. Moreover, FFRML closely reproduces FFRCFD calculations, assesses the hemodynamic severity of coronary stenosis, correlating with invasive FFR results, and improves diagnostic accuracy and positive-predictive value of CCTA on a per-vessel and per-patient level.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “CMR, thanks to phase-contrast sequences and the possibility of obtaining specific anatomic planes, has been used to evaluate valves’ anatomy. AI has been applied in this setting to classify and grade valve diseases. Fries and colleagues created a DL model that satisfactorily classified aortic valve malformations from phase-contrast CMR images. They used weak supervision to train a DL model and used it to classify bicuspid aortic valve in unlabeled MR imaging sequences from the UK Biobank. Using health outcome data, they found that the model identified individuals at increased risk of MACE. In addition, ML models have been used to identify different phenotypes of bicuspid valve-associated aortopathy (root, ascending, and arch) and their association with specific clinical findings.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “The significant number of imaging markers of CV risk, such as CAC score, plaque features, adipose tissue, and radiomics, are being incorporated to traditional clinical risk factors, in order to create predictive models that can increase the performance of current risk score and prognostic models. AI plays an important role in this process, enabling the consideration of clinical and imaging data together while including a larger number of clinical parameters. Several fusion models have been developed to combine multiple resources, and they have been successfully applied to CVD risk and severity assessment, acute CVD detection, and CVD phenotyping.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488 - “In summary, AI has been successfully used to perform time-consuming tasks, such as segmentation and postprocessing, optimization of data acquisition and reconstruction, and grading of disease severity. AI has been proven to show an improvement, in terms of time and accuracy, of human work, and therefore, serves as an aid to physicians in better understanding of the patient’s cardiac health. Several AI applications in cardiac imaging demonstrate human-level performance, and it is likely that, in the near future, these applications will be further improved to be integrated into clinical workflow; this will have a great impact on costs, wider usability, and optimization of workflow efficiency.”
The Role of Artificial Intelligence in Cardiac Imaging
Carlotta Onnis et al.
Radiol Clin N Am 62 (2024) 473–488
- “The purpose of this study was to systematically create a radiomics dataset of normal abdominal and pelvic radiomics that can be used for model development and validation. Young adults without any previously known disease, aged > 17 and ≤ 36 years old, were retrospectively included. All patients had undergone CT scanning for emergency indications. In case abnormal findings were identified, the relevant anatomical structures were excluded. Deep learning was used to automatically segment the majority of visible anatomical structures with the TotalSegmentator model as applied in 3DSlicer. Radiomics features including first order, texture, wavelet, and Laplacian of Gaussian transformed features were extracted with PyRadiomics. A Github repository was created to host the resulting dataset. Radiomics data were extracted from a total of 531 patients with a mean age of 26.8 +/- 5.19 years, including 250 female and 281 male patients. A maximum of 53 anatomical structures were segmented and used for subsequent radiomics data extraction. Radiomics features were derived from a total of 526 non-contrast and 400 contrast-enhanced (portal venous) series. The dataset is publicly available for model development and validation purposes.”
Developing a Radiomics Atlas Dataset of normal Abdominal and Pelvic computed Tomography (RADAPT)
Elisavet Kapetanou et al.
Journal of Imaging Informatics in Medicine https://doi.org/10.1007/s10278-024-01028-7 - “Organs and anatomic structures of the reproductive system not detected by TotalSegmentator have not been included. Segmentation included abdominal organs (the liver, spleen, pancreas, adrenals, kidneys, gallbladder), muscles (paraspinal muscles, gluteal muscles, iliopsoas muscles), bones (lower ribs included in abdominal images, lower thoracic vertebrae, lumbar vertebrae, pelvic bones, and proximal femurs), and vessels (aorta and common iliac arteries, portal vein, inferior vena cava, and common iliac veins).”
Developing a Radiomics Atlas Dataset of normal Abdominal and Pelvic computed Tomography (RADAPT)
Elisavet Kapetanou et al.
Journal of Imaging Informatics in Medicine https://doi.org/10.1007/s10278-024-01028-7 - “There are some inherent limitations in this study. The dataset is derived from a specific age group of young adults limited to ≥ 17 and ≤ 36 years old, which may not be entirely representative of the broader population. Age-related changes in organ morphology and tissue characteristics might introduce variability when applying this radiomics reference atlas to older populations. Nonetheless, this specific age range was chosen by design with the goal of representing healthy tissues and organs. Another limitation is that there is a possibility that an undiagnosed/ undocumented underlying disease could be present in some of our patients. Nonetheless, a comprehensive analysis of all available patient data was done to ensure that no relevant disease was on record and that no visible abnormal imaging finding was included.”
Developing a Radiomics Atlas Dataset of normal Abdominal and Pelvic computed Tomography (RADAPT)
Elisavet Kapetanou et al.
Journal of Imaging Informatics in Medicine https://doi.org/10.1007/s10278-024-01028-7
- “Renal cancer is responsible for over 100,000 yearly deaths and is principally discovered in computed tomography (CT) scans of the abdomen. CT screening would likely increase the rate of early renal cancer detection, and improve general survival rates, but it is expected to have a prohibitively high financial cost. Given recent advances in artificial intelligence (AI), it may be possible to reduce the cost of CT analysis and enable CT screening by automating the radiological tasks that constitute the early renal cancer detection pipeline. This review seeks to facilitate further interdisciplinary research in early renal cancer detection by summarizing our current knowledge across AI, radiology, and oncology and suggesting useful directions for future novel work.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “Deep learning-based classifiers can achieve high accuracy in CT images with very little manual intervention. Tanaka et al. (2020) sought to quantify small (≤4 cm) renal mass detection accuracy in CT using axial CT slices and a fine-tuned InceptionV3 CNN; they differentiated malignant and benign masses with a maximum AUC of 0.846 in CECT and 0.562 in NCCT. Pedersen et al. (2020) trained a similar 2D slice-classifying CNN, but used it to classify each slice within each known mass’ 3D volumes to enable a slice-based voting system to differentiate patient-level RC from oncocytoma, returning a perfect validation accuracy of 100%. Han et al. (2019) sought to differentiate between clear cell RCC (ccRCC) and non-ccRCC from known RCC masses, using radiologist-selected axial CT slices from NCCT and two CECT phases, and achieved sub-type classification AUCs between 0.88 and 0.94 in an internal testing dataset.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “Given the potential for RC early detection in LDCT, there is a need for more research quantifying RC segmentation performance in LDCT. Investigations into general NCCT segmentation have shown that using synthetic contrast enhancement as an auxiliary training task in MTL can improve segmentation accuracy. Therefore, an investigation in renal LDCT segmentation may be improved by introducing synthetic enhancement to CECT as an auxiliary learning task in MTL. Such an investigation would likely be complicated by Standley et al. (2020) findings – that MTL task relationships can be unique to each configuration of network architecture, hyperparameters, and dataset domain.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “This manuscript highlights and summarizes existing AI method in RC diagnosis and suggests how these can be repurposed to enable RC early detection. After summarizing existing segmentation, classification, and other AI methods in RC diagnosis, a review of analogous cancer detection and diagnosis methods across broader cancer literature and computer vision was conducted. Contrasting the RC-specific workflows to their equivalents across computer vision and other cancer domains allowed the generation of novel RC-specific research proposals that may enable AI-based RC early detection.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9
Kidney
- “Renal cancer is responsible for over 100,000 yearly deaths and is principally discovered in computed tomography (CT) scans of the abdomen. CT screening would likely increase the rate of early renal cancer detection, and improve general survival rates, but it is expected to have a prohibitively high financial cost. Given recent advances in artificial intelligence (AI), it may be possible to reduce the cost of CT analysis and enable CT screening by automating the radiological tasks that constitute the early renal cancer detection pipeline. This review seeks to facilitate further interdisciplinary research in early renal cancer detection by summarizing our current knowledge across AI, radiology, and oncology and suggesting useful directions for future novel work.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “Deep learning-based classifiers can achieve high accuracy in CT images with very little manual intervention. Tanaka et al. (2020) sought to quantify small (≤4 cm) renal mass detection accuracy in CT using axial CT slices and a fine-tuned InceptionV3 CNN; they differentiated malignant and benign masses with a maximum AUC of 0.846 in CECT and 0.562 in NCCT. Pedersen et al. (2020) trained a similar 2D slice-classifying CNN, but used it to classify each slice within each known mass’ 3D volumes to enable a slice-based voting system to differentiate patient-level RC from oncocytoma, returning a perfect validation accuracy of 100%. Han et al. (2019) sought to differentiate between clear cell RCC (ccRCC) and non-ccRCC from known RCC masses, using radiologist-selected axial CT slices from NCCT and two CECT phases, and achieved sub-type classification AUCs between 0.88 and 0.94 in an internal testing dataset.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “Given the potential for RC early detection in LDCT, there is a need for more research quantifying RC segmentation performance in LDCT. Investigations into general NCCT segmentation have shown that using synthetic contrast enhancement as an auxiliary training task in MTL can improve segmentation accuracy. Therefore, an investigation in renal LDCT segmentation may be improved by introducing synthetic enhancement to CECT as an auxiliary learning task in MTL. Such an investigation would likely be complicated by Standley et al. (2020) findings – that MTL task relationships can be unique to each configuration of network architecture, hyperparameters, and dataset domain.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9 - “This manuscript highlights and summarizes existing AI method in RC diagnosis and suggests how these can be repurposed to enable RC early detection. After summarizing existing segmentation, classification, and other AI methods in RC diagnosis, a review of analogous cancer detection and diagnosis methods across broader cancer literature and computer vision was conducted. Contrasting the RC-specific workflows to their equivalents across computer vision and other cancer domains allowed the generation of novel RC-specific research proposals that may enable AI-based RC early detection.”
Artificial intelligence for early detection of renal cancer in computed tomography: A review
William C. McGough et al.
Cambridge Prisms: Precision Medicine,1, e4, 1–9
- AIM: To evaluate renal volume and attenuation changes in patients with sepsis on contrast enhanced computed tomography (CT) with respect to the severity of sepsis
CONCLUSION: In patients with sepsis, the renal volume increases and the CT attenuation value decreases in proportion to the severity of sepsis. The changes may lag behind the peak of severity of sepsis and can be observed for a relatively long time after a patient’s recovery from sepsis.”
Sepsis patients' renal manifestation on contrast-enhanced CT.
Sasaguri K, Yamaguchi K, Nakazono T, Mizuguchi M, Irie H.
Clin Radiol. 2016 Jun;71(6):617.e1-71 - The principal cause of the renal volume increase in the present patient population was oedema likely due to capillary leakage and/or cellular infiltration by the systemic effects of sepsis; however, bilateral enlarged kidneys is not a specific finding. Other diseases, such as diabetic nephropathy, glomerulonephritis, acute interstitial nephritis (drug-induced, etc.), leukaemia, bilateral acute pyelonephritis, and hydronephrosis, should be taken into consideration when this finding is observed in patients with sepsis.
Sepsis patients' renal manifestation on contrast-enhanced CT.
Sasaguri K, Yamaguchi K, Nakazono T, Mizuguchi M, Irie H.
Clin Radiol. 2016 Jun;71(6):617.e1-71 - In conclusion, an increase in renal volume and a decrease in renal enhancement on contrast-enhanced CT were observed in patients with sepsis in proportion to the severity of the sepsis. The changes may lag behind the peak of severity of sepsis and can be observed for a relatively long time after a patient’s recovery from sepsis.
Sepsis patients' renal manifestation on contrast-enhanced CT.
Sasaguri K, Yamaguchi K, Nakazono T, Mizuguchi M, Irie H.
Clin Radiol. 2016 Jun;71(6):617.e1-71 - “The aim of this article is to illustrate the controversial CT hypoperfusion complex in patients with septic shock, characterized by the following imaging features: decreased enhancement of the viscera; increased mucosal enhancement; luminal dilation of the small bowel; mural thickening and fluid-filled loops of the small bowel; the halo sign and flattening of the inferior vena cava; reduced aortic diameter; peripancreatic oedema; abnormal parenchymal perfusion; and other controversial findings that are variably associated with each other and reversible during the early stages. Increasing physicians’ awareness of the significance of these findings could prompt alternative approaches to the early assessment and management of septic shock. In this perspective, CT imaging represents a useful tool for a complete, rapid and detailed diagnosis of clinically suspected septic shock, which can be used to improve patient outcomes.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “The diagnostic accuracy of total-body computed tomography (CT) has been well established for the identification of septic shock, allowing for a rapid and simultaneous study of multiple body areas, generating detailed and panoramic images. The aim of this article is to review the characteristics of septic shock from an imaging perspective, beyond the underlying causes and to highlight how CT can be used to identify a variety of septic shock-related signs that are collectively described as CT hypoperfusion complex. The latter describes a set of widely reported signs and symptoms that are commonly observed during trauma-associated hypovolaemic shock and can be used to identify septic shock. The early recognition, diagnosis, and treatment of septic shock have profound prognostic and therapeutic implications.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “In addition to being used to identify the underlying cause of the septic state, CT is required for the early recognition of shock-associated CT imaging signs, collectively referred to as CT hypoperfusion complex, which can improve patient prognosis and management. The CT hypoperfusion complex is frequently associated with hypotension, which can also present in many no sepsis related clinical conditions, such as trauma-induced hypotensive shock (e.g. severe head or spine injury), cardiac arrest, and diabetic ketoacidosis. The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Di Serafino M, et al.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “These signs include the decreased enhancement of the viscera, the increased mucosal enhancement and luminal dilation of the small bowel, the mural thickening and identification of fluid-filled loops in the small bowel, the halo sign and flattening of the inferior vena cava (IVC), reduced aortic diameter, peripancreatic oedema and other controversial parenchymal and visceral findings and ascites that can occur in varying combinations and are often and reversible during early stages. The presence of 2 or more vascular, visceral, or parenchymal signs is necessary to establish the presence of CT hypoperfusion complex.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Di Serafino M, et al. .
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs. These signs include the decreased enhancement of the viscera, the increased mucosal enhancement and luminal dilation of the small bowel, the mural thickening and identification of fluid-filled loops in the small bowel, the halo sign and flattening of the inferior vena cava (IVC), reduced aortic diameter, peripancreatic oedema and other controversial parenchymal and visceral findings and ascites that can occur in varying combinations and are often and reversible during early stages. The presence of 2 or more vascular, visceral, or parenchymal signs is necessary to establish the presence of CT hypoperfusion complex.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Di Serafino M, et al.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. 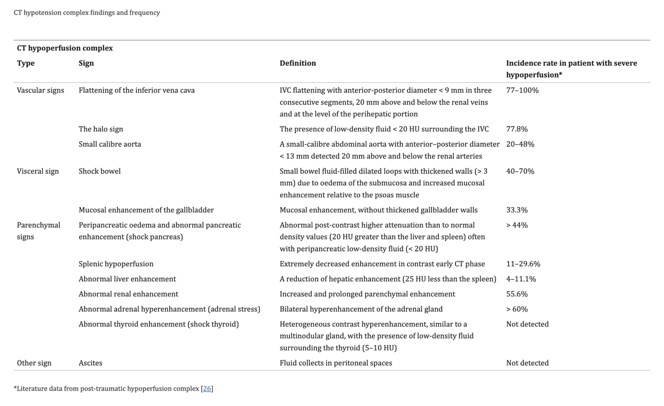
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660.- “Abnormal renal perfusion typically manifests as an increased and prolonged parenchymal enhancement; however, focal and heterogeneous enhancement can also be observed. A fall in systolic pressure causes intense efferent glomerular arteriolar vasoconstriction, which drives glomerular filtration, leading to tubular stasis and the increased resorption of salt and water. Renal parenchymal enhancement is dependent on several factors, including cardiac output and scans timing relative to the injection of contrast agent and, thus, is a non-specific sign. However, kidney enhancement can vary depending on the severity of systemic hypotension. In some cases, unlike hyperenhancement, the decreased enhancement of the renal medulla can be observed in the venous phase, likely due to the impairment of contrast medium outflow from the renal cortex to the medulla, induced by acute renal tubular dysfunction and associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - The bilateral hyperenhancement of the adrenal gland is more common in paediatric cases than in adults and can also present in combination with acute adrenal haemorrhage, which most commonly affects the right side unilaterally, with a homogeneous increase in the size of the gland and the associated suffusion of fat around the adrenal gland. Bilateral adrenal hyperenhancement is the manifestation of adrenergic mechanisms that enhance the blood flow to the vital organs. In the arterial phase, the central zone of the adrenal gland shows less intense enhancement than the peripheral zone or presents a mosaic appearance due to the heterogeneous enhancement of the central zone. In both cases, in the venous phase, the whole adrenal gland is homogenously enhanced. This sign highlights the central role played by the adrenal glands in mediating the sympathetic response to hypotensive shock and is associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex". A pictorial essay.
Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. - “In previously published studies, CT hypoperfusion complex has been almost exclusively focused on trauma-induced hypotensive shock and only few studies correlated these signs with prognosis. Some studies have suggested that flattering of IVC, shock bowel, impaired renal enhancement and splenic hypoperfusion are the most suggestive signs of hypoperfusion complex, strongly correlated with a poor prognosis although in a series of trauma-related hypovolemic shock. In contrast, only adrenal hyperenhancement has been correlated with a poor prognosis in septic shock. Despite these contradictory reports, understanding these findings could prompt the development of alternative approaches for the early assessment and management of septic shock in the emergency setting. Therefore, in addition to the application of CT for determining the underlying cause of the septic state, clinicians should be aware and be able to recognize the various CT findings that are suggestive of the hypotensive state. In this perspective, CT imaging represents a useful tool for a complete, rapid, and detailed diagnosis of clinically suspected septic shock, which can be used to improve patient outcomes.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5.
Pancreas
- Background: Pancreatic adenocarcinoma (PC) is a highly lethal malignancy with a survival rate of only 12%. Surveillance is recommended for high-risk individuals (HRIs), but it is not widely adopted. To address this unmet clinical need and drive early diagnosis research, we established the Pancreatic Cancer Early Detection (PRECEDE) Consortium.
Conclusions: PRECEDE provides infrastructure support to increase access to clinical surveillance for HRIs worldwide, while aiming to drive early PC detection advancements through longitudinal standardized clinical data, imaging, and biospecimen captures. Increased cyst prevalence in HRIs with FPC suggests that FPC may infer distinct biological processes. To enable the development of PC surveillance approaches better tailored to risk category, we recommend adoption of subclassification of HRIs into FPC, PGV1/FPC1, and PGV1/FPC– risk groups by surveillance protocols.
The Pancreatic Cancer Early Detection (PRECEDE) Study is a Global Effort to Drive Early Detection: Baseline Imaging Findings in High-Risk Individuals
George Zogopoulos et al.
J Natl Compr Canc Netw. 2024 Apr;22(3):158-166 - “PRECEDE is building a large-scale research resource with depth to advance the field of early PC detection, while improving access to PC surveillance for at-risk individuals worldwide. Our interim analysis suggests that classifying HRIs into FPC, PGV1/FPC1, and PGV1/FPC– risk groups may have biologic relevance with clinical significance for tailored surveillance of HRIs. We recommend that this subclassification of HRIs be adopted by surveillance protocols moving forward.”
The Pancreatic Cancer Early Detection (PRECEDE) Study is a Global Effort to Drive Early Detection: Baseline Imaging Findings in High-Risk Individuals
George Zogopoulos et al.
J Natl Compr Canc Netw. 2024 Apr;22(3):158-166 - “IPMNs are macroscopic lesions that develop within the pancreatic ductal system . These lesions are characterized by their cystic nature, mucin secretion and are less common than PanINs. Studies on HRIs indicate that cystic lesions, particularly branch-duct (BD) type IPMN, are the most commonly diagnosed abnormalities during pancreatic cancer surveillance. This occurrence is not unusual, considering the high prevalence of IPMNs, which can reach up to 11.3–25% among the general population aged ≥ 55 years and increases with age. Additionally, there is evidence suggesting that among HRIs, the cumulative incidence of IPMNs is even higher, exceeding 46% . Like PanINs, IPMNs are recognized as precancerous lesions and only a minority progress into PDAC. The ones that do progress, advance from low-grade IPMN to PDAC in approximately 6 years. The rate of progression is associated to the location of the lesion Based on their location, IPMNs can be categorized into three groups: main duct (MD), branch duct (BD), and mixed type (MT) involving both locations. Individuals with MD and MTIPMNs have a relative or absolute indication for surgery in the presence of specific risk factors, as outlined in the European evidence-based guidelines.”
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print. - “Pancreatic ductal adenocarcinoma (PDAC) is a major health problem with a growing incidence. It is anticipated that it will become the second-leading cause of cancer-related deaths by 2030. PDAC has a poor prognosis with a 5-year survival rate of less than 10% . While surgical resection is the only curative treatment, 80% of PDACs are unresectable at presentation. This underscores the necessity for early detection. Nonetheless, pancreatic cancer screening of the general population is not feasible due to the relatively low incidence of this disease, with 13.3 cases per 100,000 individuals per year. Even a highly accurate test would result in many false-positively diagnosed individuals, who would be subjected to unnecessary harm including operation, excessive psychological burden following from screening, and would lead to high costs.”
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print. - “Interestingly, it is known that up to 10% of individuals with PDAC have an underlying genetic predisposition. The incidence of PDAC among these high-risk individuals (HRIs) is higher compared to that of the general population, making surveillance more appropriate in that subpopulation. Although data on long-term screening results are recently starting to be published, several questions persist. It remains unclear whether surveillance results in an increased resection rate of PDAC, avoids an excessive number of unnecessary surgeries and improves survival outcomes compared to no surveillance.”
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print. - “In 2018, Canto et al. conducted a study on a surveillance cohort of 354 individuals in the United States. The cohort mainly consisted of individuals with FPC (n = 297) and the remaining 57 individuals were carriers of a genetic PV. Notably, 41 of these individuals carried a PV in BRCA1/ BRCA2, or PALB2 genes and the entire cohort included only individuals with at least six months of follow-up. The mean age of the cohort was 56.4 years and the median follow-up was 5.6 years. During the entire follow-up period a total of 10 (2.8%) PDAC cases were identified with a resection rate of 90% and a three year survival of 85%. Considering the diagnostic yield of the surveillance program, 1 (10%) stage I PDAC, 6 HGD-IPMNs and 4 PanINs-3 were detected during surveillance. In this study, 23/354 (6.5%) individuals underwent surgery for suspected malignancy, only to find out that the lesions were benign. All PDAC cases occurred in individuals with FPC, whose age ranged from 46 to 79 years old. “
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print. - “Moreover, while a pancreatic cancer surveillance program captures numerous cystic lesions, the limited ability to differentiate between malignant and benign cysts still leads to the unnecessary resection of benign cysts. Genomic based biomarkers show significant promise in addressing this challenge. In a multicenter study investigating targeted next-generation sequencing using pancreatic cyst fluid, a sensitivity of 88% and a specificity of 98% were observed in detecting the presence of advanced neoplasia in these cysts. Currently, surveillance relies solely on imaging, which is suboptimal. In the future, complementing biomarkers will enhance the early detection of PDAC in HRIs.”
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print. - “Additionally, guidelines do not provide a recommendation regarding the age at which surveillance should be discontinued. Establishing a stopping age is necessary to maintain the effectiveness of surveillance and minimize the burden. However, as every individual ages differently, with variations in fitness and health, determining a universal cutoff age is unrealistic. Reasons to discontinue surveillance, include limited life expectancy and potential risks associated with the procedures. Elderly are more likely to die from non-cancer-related causes and would subsequently no longer benefit from early PDAC detection. Moreover, patients must meet a certain level of physical fitness to undergo procedures, otherwise early PDAC detection may not yield significant benefits, as disease treatment will become unfeasible. More research needs to be conducted in this field to offer guidance on what criteria to consider in assessing the added value of surveillance in older individuals or potentially develop a prediction model that can determine whether an individual will benefit from surveillance or not.”
Aspects and outcomes of surveillance for individuals at high‑risk of pancreatic cancer
Aleksander M. Bogdanski et al.
Fam Cancer. 2024 Apr 15. doi: 10.1007/s10689-024-00368-1. Online ahead of print.

- “PNETs constitute up to 3% of clinically detected pancreatic tumors. Differentiating PNET from IPAS on CT is challenging as both may appear as well-circumscribed arterial phase enhancing lesions. Cinematic rendering is a 3D postprocessing technology that uses an advanced global lighting mode to generate photorealistic images. The precision with which cinematic rendering simulates intricate interactions of light passing through imaged volumes yields superb surface detail and textural perception.”
Cinematic Rendering for Differentiation of Pancreatic Neuroendocrine Tumor From Intrapancreatic Accessory Spleen.
Ahmed TM, Fishman EK.
AJR Am J Roentgenol. 2024 Mar;222(3):e2430862.
- The early detection of pancreatic cancer is a critical factor in improving patient outcomes, as it is often diagnosed at an advanced stage when treatment options are limited. AI has the potential to aid in the early detection of pancreatic cancer by analyzing medical data and identifying patterns that may indicate the presence of the disease. Deep learning techniques can be trained on large datasets to accurately identify early stage pancreatic cancer based on characteristic imaging features or use morphology features to build segmentation frameworks for the pancreas. AI algorithms can integrate various patient data, such as age, family history, lifestyle factors, and medical history, to detect an individual’s developing pancreatic cancer early. AI can also analyze a patient’s electronic health records, including medical history, laboratory results, and diagnostic reports, to identify potential indicators of pancreatic cancer. By processing and interpreting vast amounts of data, AI algorithms can detect subtle patterns and abnormalities that may go unnoticed by clinicians.
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; 
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051;- “The lack of large, centralized datasets that can be used to build and test algorithms poses a barrier to developing comprehensive models. Currently, there is only one major effort in addressing this through the NIH-NCI-sponsored EDRN project for pancreatic cancer. Studies that have used smaller available datasets have not accounted for suboptimal image quality and factors that make images unsuitable for AI, such as posttreatment status and the presence of biliary stents. These gaps in the quality of the data used to develop models may result in errors and biases that limit their applications in clinical medicine.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “AI can serve as a powerful tool in the advancement of pancreatic cancer diagnosis, management, and prognosis, particularly in identifying tumors earlier in disease progression. Despite the many applications and advantages of AI in pancreatic cancer, multiple limitations pose challenges that must be addressed as the field grows. One is the lack of a standardized approach to treatment and diagnosis. Other challenges include a lack of robust and high-quality data, transparency and reproducibility of findings, and ethical considerations, including biases in algorithms.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - Furthermore, AI algorithms have been previously referred to as “Black boxes” due to their lack of transparency and interpretability. The opacity of the code used to build AI models and the hidden level of complexity make it difficult to reproduce results in an independent manner. General descriptions of the code used to build models do not provide enough information to reproduce most findings. The lack of easy interpretation of these AI models and prospective studies assessing AI-based tools has increased the hesitancy of adaptation into clinical practice. Without transparency and interpretation, clinicians are not able to critically interrogate the output of these models, putting an incredible amount of faith in the accuracy of the model. Improving reproducibility and interpretability will be crucial challenges to overcome prior to the clinical adaptation of AI models in pancreatic cancer.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Lastly, a few ethical concerns should be considered when discussing the implementation of AI in pancreatic cancer. Datasets used to build models tend to lack data from underrepresented groups such as women and minorities, leading to biased models that may not be applicable to the diverse patient population seen clinically. Implementing these skewed algorithms can increase disparities in health outcomes between groups rather than improving outcomes, particularly because models tend to perform best on data that are most like the data they were trained with. Improving the diversity in patient data used to train models and validating models across various populations could mitigate this challenge and provide models that are more generalizable to a heterogeneous patient population. Additionally, the creation and use of large datasets needed to create AI models pose the challenging questions of data ownership and patient privacy, particularly in reference to medical imaging. At the same time, the integration of AI systems in medical practices raises questions about the security and confidentiality of sensitive patient data. Ensuring robust data protection mechanisms is imperative to prevent unauthorized access and potential misuse of personal health information.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Pancreatic cancer is a complicated disease with molecular heterogeneity. Integrating multi-omics data, such as genomes, transcriptomics, proteomics, and metabolomics, can offer a complete picture of the disease pathology. Future research should concentrate on building artificial intelligence algorithms capable of assessing and combining these disparate datasets in order to uncover strong molecular signatures, biomarkers, and therapeutic targets for pancreatic cancer.”
From Machine Learning to Patient Outcomes: A Comprehensive Review of AI in Pancreatic Cancer.
Tripathi S, et al.
Diagnostics (Basel). 2024 Jan 12;14(2):174. doi: 10.3390/diagnostics14020174. PMID: 38248051; - “Current guidance for pancreatic cancer surveillance is restricted to high-risk individuals (HRIs) who have germline mutations that predispose to a lifetime increased risk of pancreatic cancer or a strong family history of pancreatic cancer. When a pancreatic cyst is detected incidentally by abdominal imaging, such patients are often put in the HRI category for surveillance. Cumulatively, HRIs account for only about 20–25% of cases. What about the majority of patients with no risk factors who present at an advanced stage? This is where advances in artificial intelligence (AI) based on mining of electronic health records (EHRs) have started to show some promise. ”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - “ It is important to emphasize that these are retrospective studies and have not yet been evaluated prospectively in a real-world setting. Nonetheless, such research provides a potential roadmap for early detection of pancreatic cancer that extends beyond the current narrow definition of HRIs by enriching the general population with a larger proportion of individuals at “sporadic” risk who are identified through the mining of EHR data (figure). If prospective studies support this approach, this enriched population could then undergo longitudinal surveillance using liquid biopsy tools (circulating tumour DNA, methylation assays, or proteins) that are being deployed in the context of early detection of multiple cancers.”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - ”The contribution of AI does not stop at the initial EHR-based enrichment step since deep learning models are also being developed to improve the resolution of CT and MRI imaging scans for the detection of early, subcentimetric cancers in the pancreas. In addition to changes within the pancreas, these computational algorithms could also identify subtle changes in body composition (eg, attenuation of visceral fat and muscle) that may be missed by clinicians. With such surveillance of a high-risk group, early diagnosis would be enabled, as would the potential for improving outcomes, with treatment including surgical resection followed by emerging immunotherapy options, such as personalised vaccines. Therein lies the opportunity for AI support to help advance diagnosis and care for pancreatic cancer. ”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682. - “ In a study that used a transformer AI model that incorporated time sequence data of longitudinal EHRs over several years, an aggregate of nearly 28 000 cases of pancreatic cancer were analyzed and compared with 11 million patients who did not develop this disease. The primary dataset was from over 6 million patients in a Danish national registry, and findings were subsequently validated in an additional 3 million patients in the US Veterans Affairs system. The authors were able define a group of people among those aged 50 years and older who had a 30–60 times higher risk than the general population of being diagnosed with pancreatic cancer within the next 12 months. One of the EHR diagnostic codes that the model consistently identified as a feature predictive of incident pancreatic cancer within the next 24 months was diabetes, reinforcing the established link between new-onset diabetes and underlying pancreatic cancer. A second independent study used AI to differentiate the approximately 35 000 patients who developed pancreatic cancer from 1·5 million people who did not. This study identified over 80 features derived from EHRs, laboratory tests, symptoms, medications, and coexisting conditions that defined increased risk. Some of the features within the algorithm are intuitive, such as age or diabetes, whereas others underscore how AI can identify patterns not readily discernible by human assessment (eg, mean corpuscular haemoglobin concentration in red blood cells).”
Early detection of pancreatic cancer and AI risk partitioning.
Maitra A, Topol EJ.
Lancet. 2024 Apr 13;403(10435):1438. doi: 10.1016/S0140-6736(24)00690-1. PMID: 38615682.
Practice Management
- “Uncertainty regarding the future of radiologists is largely driven by the emergence of artificial intelligence (AI). If AI succeeds, will radiologists continue to monopolize imaging services? As AI accuracy progresses with alacrity, radiology reads will be excellent. Some articles show that AI can make non-radiologists experts. However, eminent figures within AI development have expressed concerns over its possible adverse uses. Bad actors, not bad AI, may account for a future in which AI is not as successful as we might hope and, as some fear, even pernicious. More relevant to current predictions over the course of AI in medicine, and radiology in particular, is how the evolution of AI is often seen in a vacuum. We cannot predict the future with certainty. But as we contemplate the potential impact of AI in radiology, we should remember that radiology does not exist in a vacuum; while AI is changing, so is everything else. The medical system, not to mention the world's population, has been severely impacted by the global COVID-19 pandemic and numerous experts expect future worldwide pandemics. We cannot predict the condition of the healthcare system in two decades but may assume that radiology will likely remain critical in any future medical practice. For now, we should responsibly use all tools at our disposal (including AI) to make ourselves as indispensable as possible. Our best chances of remaining relevant and instrumental to patient care will likely hinge on our ability to lead the changes rather than be passively impacted by them.”
The future of radiology and radiologists: AI is pivotal but not the only change afoot.
Weisberg EM, Fishman EK.
J Med Imaging Radiat Sci. 2024 Feb 24:S1939-8654(24)00016-X. - “We cannot predict the future with certainty. But as we contemplate the potential impact of AI in radiology, we should remember that radiology does not exist in a vacuum; while AI is changing, so is everything else. The medical system, not to mention the world's population, has been severely impacted by the global COVID-19 pandemic and numerous experts expect future worldwide pandemics. We cannot predict the condition of the healthcare system in two decades but may assume that radiology will likely remain critical in any future medical practice. For now, we should responsibly use all tools at our disposal (including AI) to make ourselves as indispensable as possible. Our best chances of remaining relevant and instrumental to patient care will likely hinge on our ability to lead the changes rather than be passively impacted by them.”
The future of radiology and radiologists: AI is pivotal but not the only change afoot.
Weisberg EM, Fishman EK.
J Med Imaging Radiat Sci. 2024 Feb 24:S1939-8654(24)00016-X.
Small Bowel
- “Small-intestine infection may be seen as symmetric and homogeneous thickening of the ileal wall, which may be focal or diffuse on CT. A feathery pattern of mucosal thickening may also be seen on ultrasound. Sometimes colonic involvement can be seen in the absence of ileal involvement and may be patchy or continuous. Salmonella enteritis may even simulate pseudomembranous colitis, with toxic megacolon as a known complication. Enlarged mesenteric nodes may be seen adjacent to the involved segment of intestine. Gastrointestinal bleeding and perforation are important complications and occur frequently in the terminal ileum. Active bleeding may be visualized in the form of intravascular contrast extravasation on CT angiography studies.”
Spectrum of Imaging Findings in Salmonella Infections
Tiffany Hennedige et al.
AJR 2012; 198:W534–W539 - “Ascites, localized or generalized mesenteric stranding, thickening, and adenopathy are frequent manifestations of Salmonella infection. Salmonella infections have a predilection for the gastrointestinal tract. Involvement of the terminal ileum or the proximal colon with mesenteric lymphadenopathy may be specific imaging findings.”
Spectrum of Imaging Findings in Salmonella Infections
Tiffany Hennedige et al.
AJR 2012; 198:W534–W539 - “A wide spectrum of radiologic manifestations due to Salmonella infection may be encountered, especially in endemic areas and immunocompromised patients. However, the imaging findings in Salmonella infection are not unique and can mimic other infective diseases. Knowledge of radiologic manifestations is important to aid in early diagnosis and timely initiation of appropriate management. In our experience, in the appropriate clinical setting, radiologic findings of thickened terminal ileum or proximal colon with mesenteric lymphadenopathy are specific for Salmonella infection.”
Spectrum of Imaging Findings in Salmonella Infections
Tiffany Hennedige et al.
AJR 2012; 198:W534–W539
Vascular
- “BBCR captures the cardiac valve anatomy and calcifications. CR has been used in the assessment of aortic valve variant anatomy, and BBCR improves the delineation of the valve cusps from the blood pool. Global valve and outflow tract calcification assessment can be challenging to fully capture on 2D imaging, and routine cinematic rendering displays often obscure the calcifications due to the opaque contrast-enhanced blood pool. BBCR has potential for the assessment of calcifications of the valve leaflets, annulus, and outflow tract in planning for transcatheter valve replacements .”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. 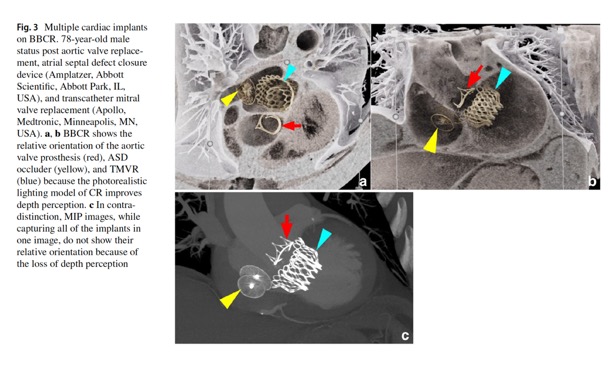
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284.

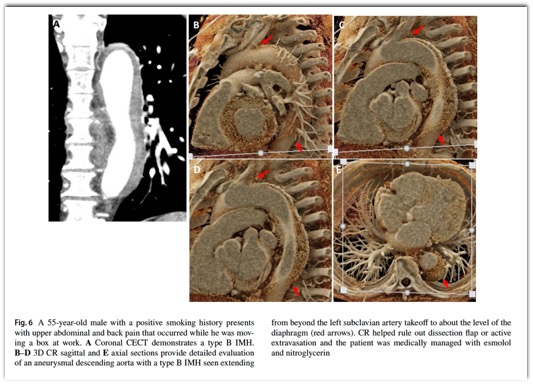
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276.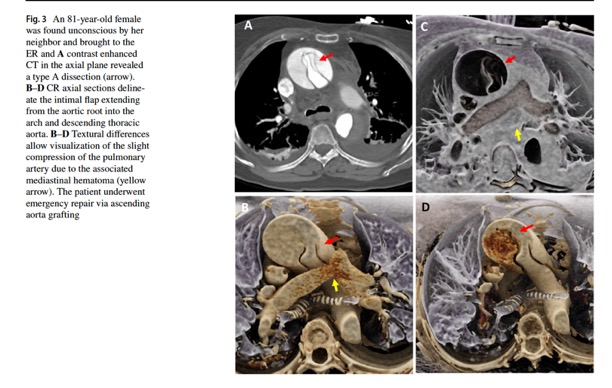
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276.- “Non-traumatic thoracic aorta emergencies are acute conditions associated with substantial morbidity and mortality. In the emergency setting, timely detection of aortic injury through radiological imaging is crucial for prompt treatment planning and favorable patient outcomes. 3D cinematic rendering (CR), a novel rendering algorithm for computed tomography (CT) image processing, allows for life-like visualization of spatial details and contours of highly complex anatomic structures such as the thoracic aorta and its vessels, generating a photorealistic view that not just adds to diagnostic confidence, but is especially useful for non-radiologists, including surgeons and emergency medicine physicians.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “CR involves global illumination and path tracing models whereby numerous light rays from all directions propagate through and interact with the volumetric data to generate a voxel. Complex anatomical relations are better evaluated and enhanced depth and shape perception is achieved as the technique considers a natural lighting environment and its effects (e.g., reflection, diffusion, refraction). Postprocessing windowing and the use of clip planes/masks allow cutting into the volume and isolation of the area/organ of interest.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “As with other 3D post-processing methods, the individual slices are stacked to create a 3D volume, upon which the specialized CR lighting model is applied. This lighting model is unique in that it simulates the propagation of millions of photos through the volume dataset, and thus creates realistic interactions with the various tissue compositions, producing photorealistic images from the dataset. This in turn results in a greater degree of surface detail and shadowing as opposed to traditional VR. Where VR techniques employ a local lighting model, the global illumination model used in CR entails complex calculations for rendering, as both direct and indirect illumination are traced, as well as the effect of diffusion, scatter, and reflections.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “There are some limitations that come with 3D CR. Notably, shadows generated in the images might conceal certain pathologies when viewed from specific angles, necessitating meticulous optimization and assessment from diverse angles in conjunction with the multiplanar reformations. Thus, while an initial learning period to become adept in handling and familiarizing themselves with the CR process is required for radiologists, as demonstrated in our case studies, an experienced radiologist can efficiently execute the rendering process in under 5 min.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “Color mapping of different phases enhances visualization of the key pathology, such as the flow through the false and true lumens in a dissection that can be delineated by high contrast shading. CR rendering emphasizes textural changes attributable to inflammatory processes with realistic shadowing that is otherwise difficult to appreciate. The improved surface detail helps characterize an impending PAU or the nature of outpouchings suspicious for mycotic aneurysms and gives a clearer view of multiple plaques and sites of ulceration that could be otherwise missed.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - Another application of 3D CR is via the black blood cinematic rendering (BBCR) preset.. BBCR is a preset we specifically developed to visualize intraluminal contours and structures of the heart and great vessels, all through adjustments that can be made in under a minute. This is especially useful in the setting of visualizing various zones of thrombi and occlusion, the degree of obstruction, and the subtle irregularities and internal arrangement of the thrombus that can only be appreciated due to enhanced depth perception and shadowing.
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “3D cinematic rendering (CR) represents an important advancement in radiological imaging, particularly in enhancing the visualization of complex anatomical structures and systems such as the thoracic aorta and its vessels. CR provides detailed, photorealistic illustrations crucial for diagnosis and surgical planning as we have seen in several cases. Future research is needed to evaluate CR’s diagnostic accuracy, both prospectively and in head-to-head comparisons with other rendering methods, as well as its role in other domains such as patient education and medical training. CR, therefore, is emerging as a promising, evolving tool for radiologists, surgeons, and the patients they treat.”
Cinematic rendering of non-traumatic thoracic aorta emergencies: a new look at an old problem.
Yasrab M, Rizk RC, Chu LC, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):269-276. - “Black blood cinematic rendering (BBCR) is a newly described preset for cinematic rendering, which creates photorealistic displays from volumetric data sets with the contrast-enhanced blood pool displayed as dark and transparent. That set of features potentially provides for enhanced visualization of endomyocardial and intraluminal pathology, as well as cardiac devices. The similarity of the images to black-blood magnetic resonance imaging (MRI) may allow for expansion of the evaluation of certain types of pathology into patient populations unable to undergo MRI. In the emergency setting, the rapid acquisition time and reasonable post-processing time make this technique clinically feasible.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “Importantly, in CR, the display color and transparency can be adjusted for each tissue component. This is analogous to window center and level adjustments for standard CT review. Typically, in CR, the contrast-enhanced blood pool is displayed as an opaque light color. Black blood cinematic rendering (BBCR) was a recently described color and transparency preset that displays the contrast-enhanced blood pool as both transparent and dark. That creates images similar to black blood cardiac magnetic resonance imaging (MRI). In our experience, BBCR excels in evaluation of cardiovascular intraluminal structures because of improved detail and delineation from the contrast-opacified blood pool.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “BBCR can be created on any cinematic rendering software by adjusting the trapezoids of the voxel histogram and color Look-Up-Table. Our cardiac BBCR preset was developed at our institution by one of the authors (EKF). It was previously reported and has been published for use . Our preset can be used in SyngoVia 30, 40, 50, and 60. We have not tried this preset on other CR programs, so it is unclear exactly how to replicate it, but theoretically, the underlying concept should apply to all trapezoid-based CR programs. The general concept is to assign 0% opacity to the Hounsfield units of the contrast-enhanced blood pool. Use a narrow-plateau trapezoid to assign color(s) and high opacity to densities less than blood pool to visualize the soft tissues forming the endoluminal walls. Use a wide plateau trapezoid to assign color(s) and high opacity to densities greater than blood pool to visualize calcifications and cardiac implants.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “Further, the images produced by BBCR, although derived from standard CT acquisitions, are also fundamentally different. The pixels on a BBCR image incorporate varying color and transparency based on the proportion of different tissue types that contribute to that pixel. As such, there may be information in BBCR images that cannot otherwise specifically be abstracted from standard reconstructions or reformations. How such images and data might feed into graphical processing unit-based artificial intelligence algorithms is not easily predictable but should be explored.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - “With the growing applications of cinematic rendering in cardiovascular imaging, BBCR is valuable for its visualization of intraluminal structures through a dark transparent blood pool. In this expanded experience, we have reviewed our initial experiences with BBCR with cardiac devices, native cardiac valves and coronaries, intracardiac masses, and aortic disease. Given the often-acute presentations of those conditions, many patients may first be evaluated in the emergency setting. Although the creation of any CR images requires a workflow with a standalone workstation and an experienced radiologist, the time to generate BBCR images would be well invested in the relatively rare patient that has an endoluminal cardiac or endovascular condition that needed to be optimally evaluated.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284. - ”Similar to intracardiac structures, intraluminal vascular structures are well seen on BBCR. Cinematic rendering has been used in the detection of acute aortic injury, and differentiation of blunt aortic injury from ductus diverticulum variant anatomy. Based on the utility of bright blood CR for the aorta, BBCR could add value in the setting of acute aortic syndrome or injury to detect subtle intraluminal irregularities. It can also be used to evaluate larger intraluminal lesions or clots and their relationship to nearby anatomy.”
Expanded experience with cardiovascular black blood cinematic rendering.
Brookmeyer C, Chu LC, Rowe SP, Fishman EK.
Emerg Radiol. 2024 Apr;31(2):277-284.
- The bilateral hyperenhancement of the adrenal gland is more common in paediatric cases than in adults and can also present in combination with acute adrenal haemorrhage, which most commonly affects the right side unilaterally, with a homogeneous increase in the size of the gland and the associated suffusion of fat around the adrenal gland. Bilateral adrenal hyperenhancement is the manifestation of adrenergic mechanisms that enhance the blood flow to the vital organs. In the arterial phase, the central zone of the adrenal gland shows less intense enhancement than the peripheral zone or presents a mosaic appearance due to the heterogeneous enhancement of the central zone. In both cases, in the venous phase, the whole adrenal gland is homogenously enhanced. This sign highlights the central role played by the adrenal glands in mediating the sympathetic response to hypotensive shock and is associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. - “In previously published studies, CT hypoperfusion complex has been almost exclusively focused on trauma-induced hypotensive shock and only few studies correlated these signs with prognosis. Some studies have suggested that flattering of IVC, shock bowel, impaired renal enhancement and splenic hypoperfusion are the most suggestive signs of hypoperfusion complex, strongly correlated with a poor prognosis although in a series of trauma-related hypovolemic shock. In contrast, only adrenal hyperenhancement has been correlated with a poor prognosis in septic shock. Despite these contradictory reports, understanding these findings could prompt the development of alternative approaches for the early assessment and management of septic shock in the emergency setting. Therefore, in addition to the application of CT for determining the underlying cause of the septic state, clinicians should be aware and be able to recognize the various CT findings that are suggestive of the hypotensive state. In this perspective, CT imaging represents a useful tool for a complete, rapid, and detailed diagnosis of clinically suspected septic shock, which can be used to improve patient outcomes.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. - Perfusion Changes in the Kidneys: Differential Dx
- Obstruction (stone, tumor)
- Infection (acute pyelonephritis)
- Vascular (occlusion, stenosis of renal artery
- Trauma (vascular or otherwise)
- Dehydration
- IVDA - “With the striated nephrogram pattern, the normally homogenous nephrogram is replaced by a mixture of alternating low-attenuation and normal-enhancing or hyperdense bands within the kidney. The bands are arranged radially, similar to the collecting ducts.”
Nephrographic and pyelographic analysis of CT urography: principles, patterns, and pathophysiology.
Wolin EA, Hartman DS, Olson JR.
AJR Am J Roentgenol. 2013 Jun;200(6):1210-4. - “The nephrographic pattern of rim nephrogram (enhancement peripherally within the subcapsular zone and centrally in the cortex immediately adjacent to the medulla) is seen almost always in the setting of acute renal artery occlusion and global infarction. It is the most specific indicator of renovascular compromise. After occlusion of the main renal artery, capsular, peripelvic, and periureteric vessels will enlarge. These collateral vessels supply the nephrons in the subcapsular and juxtamedullary regions, surrounding an otherwise nonfunctioning kidney.”
Nephrographic and pyelographic analysis of CT urography: principles, patterns, and pathophysiology.
Wolin EA, Hartman DS, Olson JR.
AJR Am J Roentgenol. 2013 Jun;200(6):1210-4. - “With the reverse rim nephrogram pattern, the only enhancement is seen centrally within the renal medulla .There is no cortical enhancement. This pattern is almost always identified in the setting of acute cortical necrosis, a rare form of acute renal failure. Cortical necrosis may result from any cause of prolonged systemic hypotension, especially obstetric complications, transfusion reaction, and transplant rejection. The exact pathophysiology of vascular redistribution is a poorly understood process.”
Nephrographic and pyelographic analysis of CT urography: principles, patterns, and pathophysiology.
Wolin EA, Hartman DS, Olson JR.
AJR Am J Roentgenol. 2013 Jun;200(6):1210-4. - “With the spotted nephrogram pattern, the normally homogeneous nephrogram is replaced by segmental and subsegmental areas of nonenhancement (Appendix 1). The pathophysiologic basis for the zones of nonenhancement is segmental (lobar) and subsegmental infarctions as a result of small-vessel occlusion. This can be seen with multiple causes of intrarenal vasculitis including polyarteritis nodosa, Wegener granulomatosis, systemic lupus erythematosus, scleroderma, and arteriolar nephrosclerosis. Less commonly, a spotted nephrogram results from multiple emboli. The most common source for renal embolic disease is the heart because of valvular heart disease or left ventricular dyskinesia, but it may also come from aortic aneurysm or plaques in severe atherosclerosis.”
Nephrographic and pyelographic analysis of CT urography: principles, patterns, and pathophysiology.
Wolin EA, Hartman DS, Olson JR.
AJR Am J Roentgenol. 2013 Jun;200(6):1210-4. 
Nephrographic and pyelographic analysis of CT urography: principles, patterns, and pathophysiology.
Wolin EA, Hartman DS, Olson JR.
AJR Am J Roentgenol. 2013 Jun;200(6):1210-4.
- “The diagnostic accuracy of total-body computed tomography (CT) has been well established for the identification of septic shock, allowing for a rapid and simultaneous study of multiple body areas, generating detailed and panoramic images. The aim of this article is to review the characteristics of septic shock from an imaging perspective, beyond the underlying causes and to highlight how CT can be used to identify a variety of septic shock-related signs that are collectively described as CT hypoperfusion complex. The latter describes a set of widely reported signs and symptoms that are commonly observed during trauma-associated hypovolaemic shock and can be used to identify septic shock. The early recognition, diagnosis, and treatment of septic shock have profound prognostic and therapeutic implications.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “In addition to being used to identify the underlying cause of the septic state, CT is required for the early recognition of shock-associated CT imaging signs, collectively referred to as CT hypoperfusion complex, which can improve patient prognosis and management. The CT hypoperfusion complex is frequently associated with hypotension, which can also present in many no sepsis related clinical conditions, such as trauma-induced hypotensive shock (e.g. severe head or spine injury), cardiac arrest, and diabetic ketoacidosis. The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “These signs include the decreased enhancement of the viscera, the increased mucosal enhancement and luminal dilation of the small bowel, the mural thickening and identification of fluid-filled loops in the small bowel, the halo sign and flattening of the inferior vena cava (IVC), reduced aortic diameter, peripancreatic oedema and other controversial parenchymal and visceral findings and ascites that can occur in varying combinations and are often and reversible during early stages. The presence of 2 or more vascular, visceral, or parenchymal signs is necessary to establish the presence of CT hypoperfusion complex.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “The CT hypoperfusion complex has important prognostic and therapeutic implications and must be promptly recognized. However, although the pathogenic mechanisms that underlie hypotensive shock and septic shock are quite different, the CT findings associated with these two syndromes are often comparable to those that have been widely described in previous literature as in post-traumatic hypotensive shock, which can be grouped into vascular, visceral, and parenchymal signs. These signs include the decreased enhancement of the viscera, the increased mucosal enhancement and luminal dilation of the small bowel, the mural thickening and identification of fluid-filled loops in the small bowel, the halo sign and flattening of the inferior vena cava (IVC), reduced aortic diameter, peripancreatic oedema and other controversial parenchymal and visceral findings and ascites that can occur in varying combinations and are often and reversible during early stages. The presence of 2 or more vascular, visceral, or parenchymal signs is necessary to establish the presence of CT hypoperfusion complex.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660. - “Abnormal renal perfusion typically manifests as an increased and prolonged parenchymal enhancement; however, focal and heterogeneous enhancement can also be observed. A fall in systolic pressure causes intense efferent glomerular arteriolar vasoconstriction, which drives glomerular filtration, leading to tubular stasis and the increased resorption of salt and water. Renal parenchymal enhancement is dependent on several factors, including cardiac output and scans timing relative to the injection of contrast agent and, thus, is a non-specific sign. However, kidney enhancement can vary depending on the severity of systemic hypotension. In some cases, unlike hyperenhancement, the decreased enhancement of the renal medulla can be observed in the venous phase, likely due to the impairment of contrast medium outflow from the renal cortex to the medulla, induced by acute renal tubular dysfunction and associated with poor prognosis.”
Computed tomography imaging of septic shock. Beyond the cause: the "CT hypoperfusion complex".
Di Serafino M, et al.
A pictorial essay. Insights Imaging. 2021 Jun 5;12(1):70. doi: 10.1186/s13244-021-01006-5. PMID: 34089401; PMCID: PMC8178660.

















