Pancreatic Cancer Imaging: A New Look at an Old Problem
Pancreatic Cancer Imaging: A New Look at an Old Problem Linda C. Chu The Russel H. Morgan Department of Radiology and Radiological Science, Department of Computer Science, Department of Pathology, and the Department of Cancer Research, Johns Hopkins University, Baltimore |
Disclosure: Research support from The Lustgarten Foundation:
|
Overview Research support from The Lustgarten Foundation:
|
Introduction
|
Current State-of-the-Art 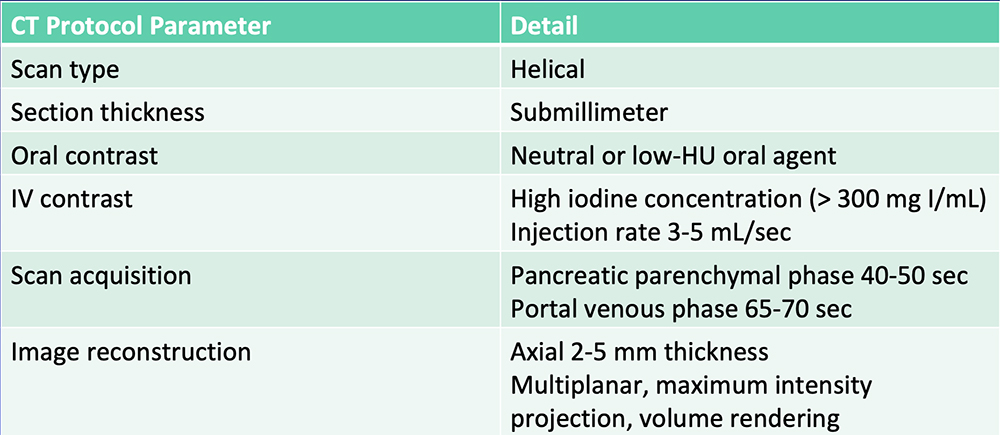 Al-Hawary MM et al. Radiology. 2014;270:248-60. |
Reporting Template 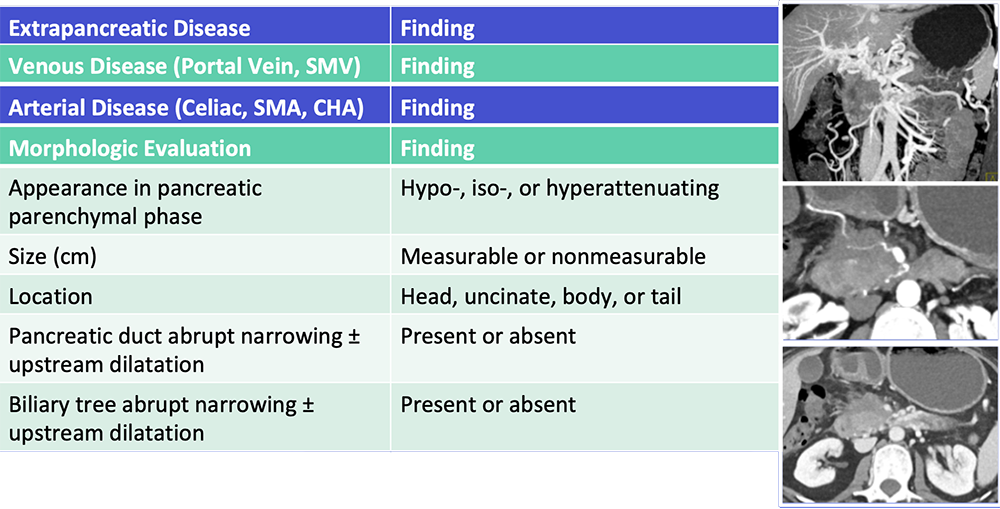 Al-Hawary MM et al. Radiology. 2014;270:248-60. Zaky AM et al. RadioGraphics. 2017;37:93-112. |
Current Frontiers for Improvement
|
Current Frontiers for Improvement Emerging imaging and analytic techniques have the potential to improve pancreatic cancer imaging 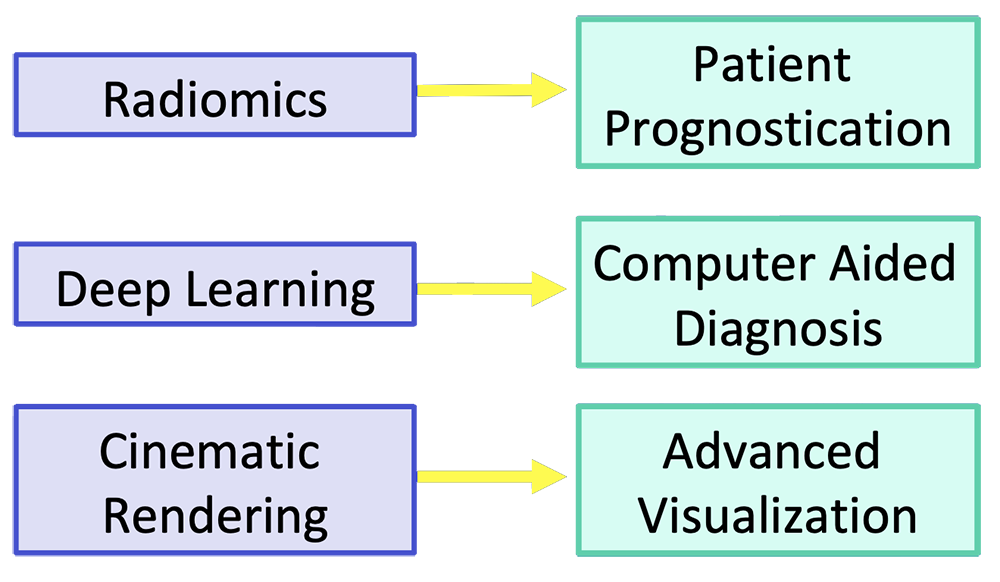 |
Patient Prognostication
|
Radiomics
Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics 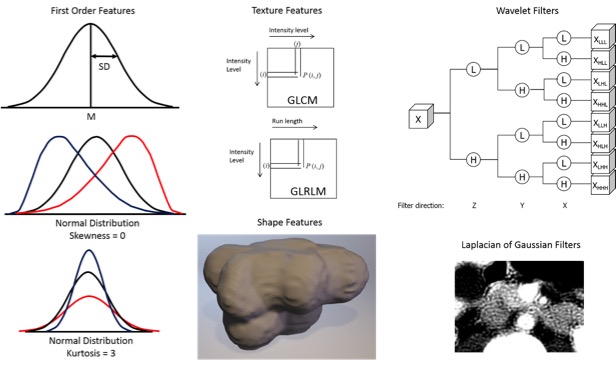 Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Aerts HJ et al. Nat Commun. 2014;5:4006. Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Survival Predication – Our Experience Retrospective study of 125 patients with surgically resected PDAC and preoperative dual-phase CT between 2010 to 2014:
|
Survival Predication – Our Experience
|
Survival Predication – Our Experience 10 radiomics features showed 82% accuracy in classification of high-risk vs. low-risk survival Radiomics features from preop CT may be useful in risk stratification prior to planned resection of PDAC 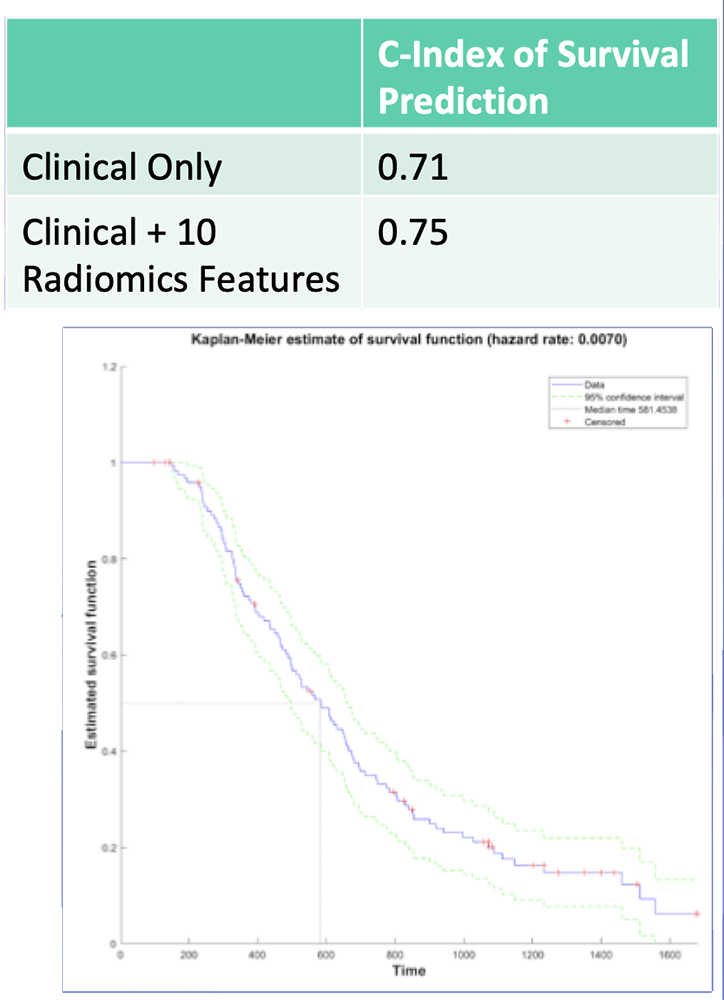 Image: Predicted Survival Curve Based on Clinical Parameters + 10 Radiomics Features |
Survival Prediction – From the Literature 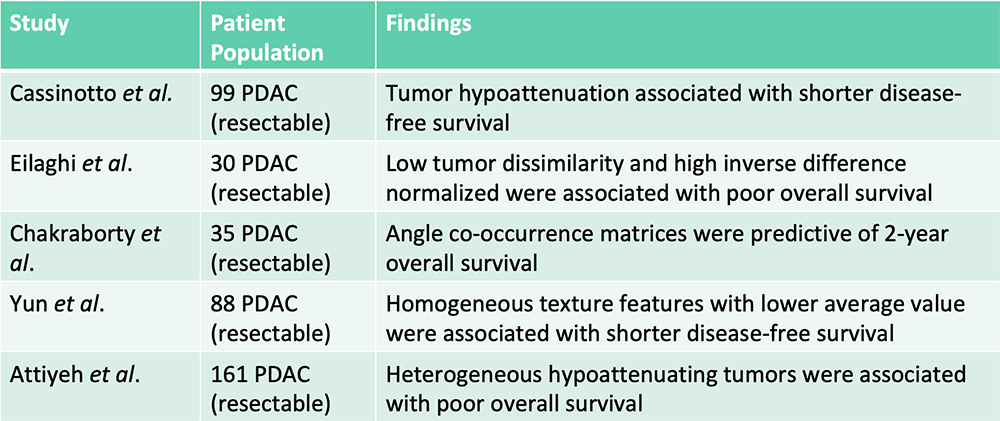 Radiomics features were additive to clinical features in predicting patient survival Cassinotto C et al. Eur J Radiol. 2017;90:152-158. Eilaghi A et al. BMC Medical Imaging. 2017;17(1):38. Chakraborty J et al. PLoS One. 2017;12(12):e0188022. Yun G et al. Scientific Reports. 2018;8:7226. Attiyeh MA et al. Ann Surg Oncol. 2018;25:1034-1042. |
Pancreatic Cyst Risk Stratification – Our Experience
|
Pancreatic Cyst Risk Stratification – Our Experience Utility of radiomics in classification of pancreatic cystic neoplasms:
|
Pancreatic Cyst Risk Stratification – Our Experience
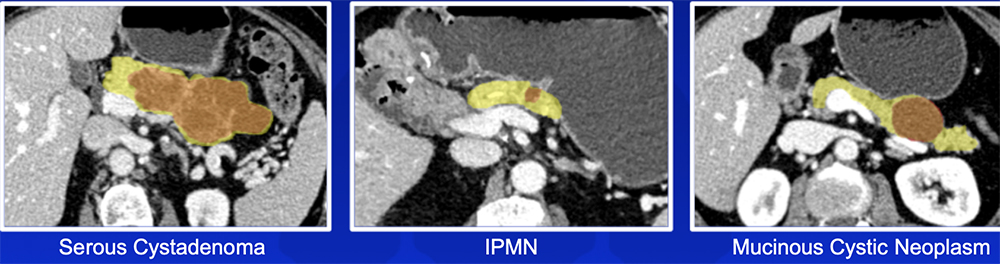 |
Risk Stratification of IPMNs – From the Literature 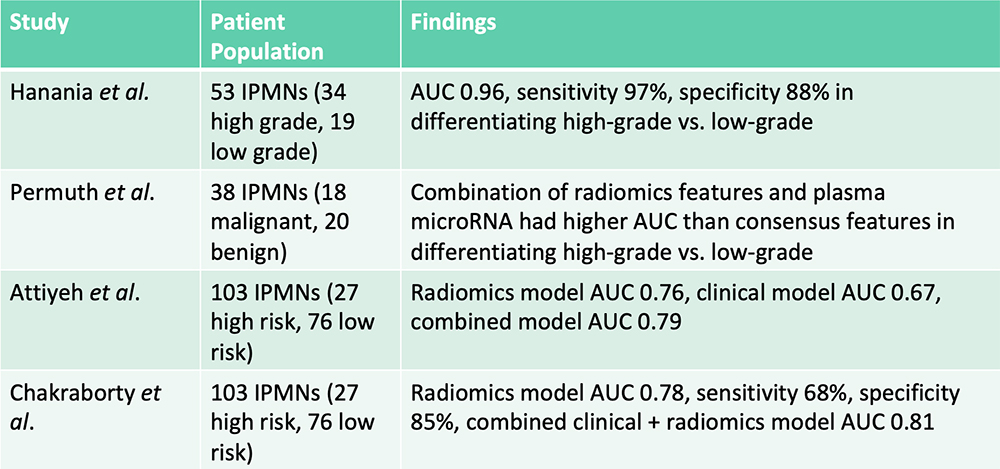 Hanania AN et al. Oncotarget. 2016;7(52):85776-85784. Permuth JB et al. Oncotarget. 2016;7(52):85785-85797. Attiyeh MA et al. HPB (Oxford). 2019;21(2):212-218.. Chakraborty J et al. Med Phys. 2018;45(11):5019-5029. |
PDAC: Opportunity for Earlier Detection
|
PDAC: Opportunity for Earlier Detection
|
Deep Learning Deep learning uses training data and multiple layers of equations to develop a mathematical model that fits the data 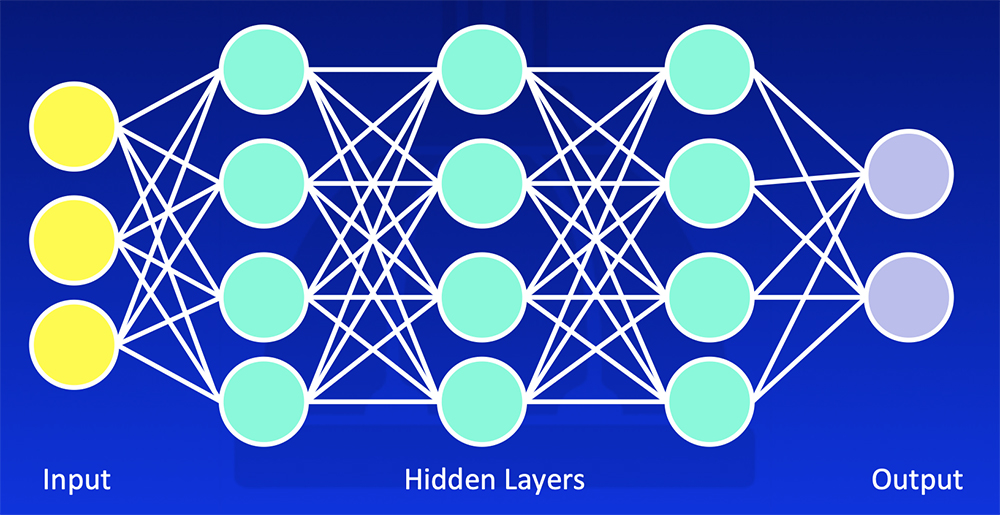 Erickson BJ et al. RadioGraphics 2017;37:505-15. Kohli M et al. AJR 2017;208:754-60 |
Deep Learning
|
Deep Learning – Our Experience
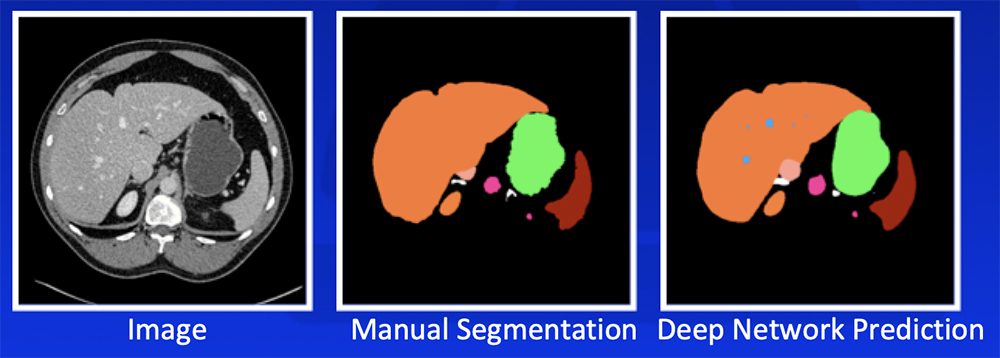 Park S et al. Diagn Interv Imaging. 2019 [Epub ahead of print]. Yan W et al. Medical Image Analysis. 2019;55:88-102 |
Deep Learning – Our Experience Our deep learning algorithm can achieve >85% accuracy in segmentation of major abdominal organs 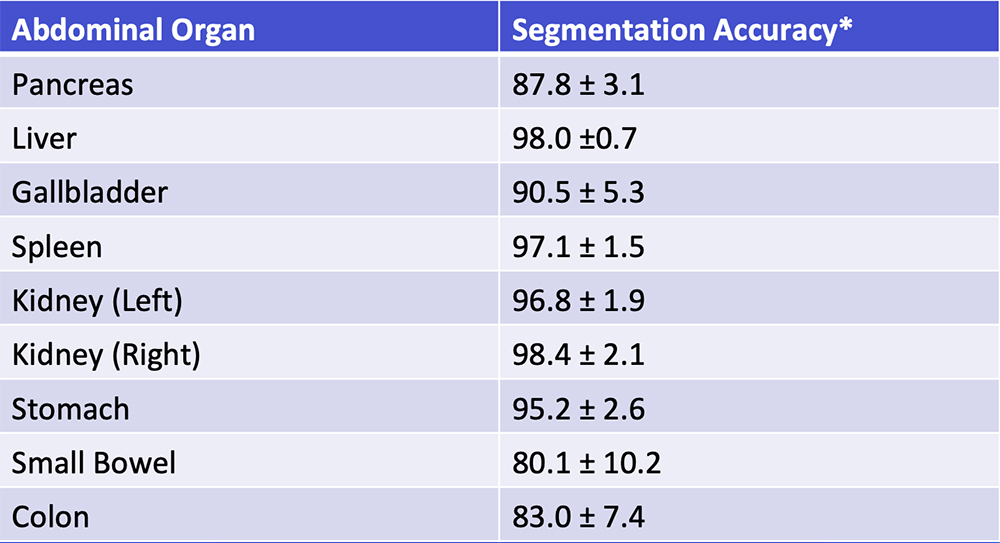 *Segmentation accuracy as defined by Dice-Sørensen coefficient Park S et al. Diagn Interv Imaging. 2019 [Epub ahead of print]. Yan W et al. Medical Image Analysis. 2019;55:88-102 |
Deep Learning – Our Experience Our deep learning algorithm can detect PDAC with >90% sensitivity and >90% specificity with a range of sizes and appearances 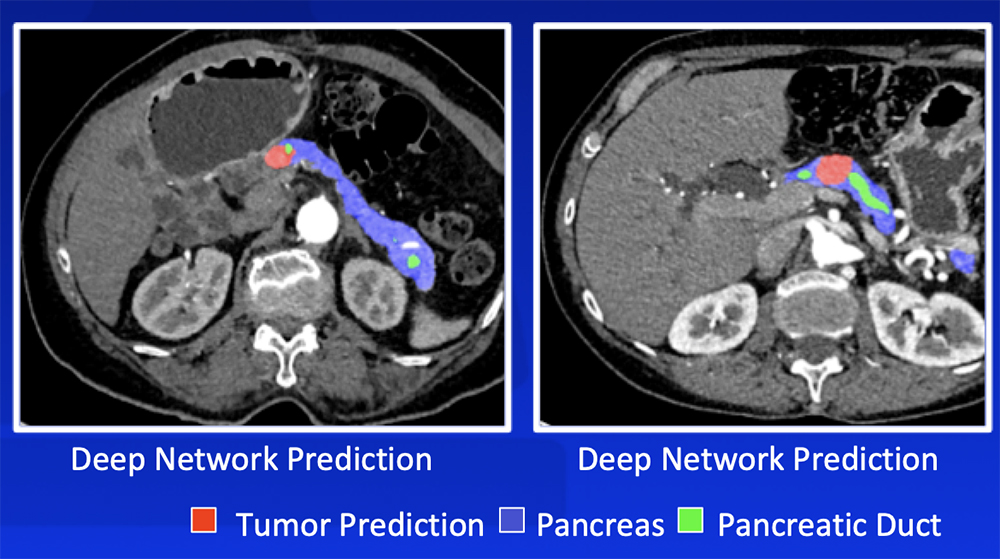 Zhu Z et al. MICCAI 2019. Chu LC et al. J Am Coll Radiol. 2019;16:1338-1342. |
Deep Learning – Ongoing Improvements
|
Advanced Visualization Techniques
|
Cinematic Rendering (CR)
Chu LC et al. Abdom Radiol (NY). 2018:43(11):3009-3015. |
Cinematic Rendering (CR) CR increases depth perception and accentuates texture differences and can potentially improve appreciation of: 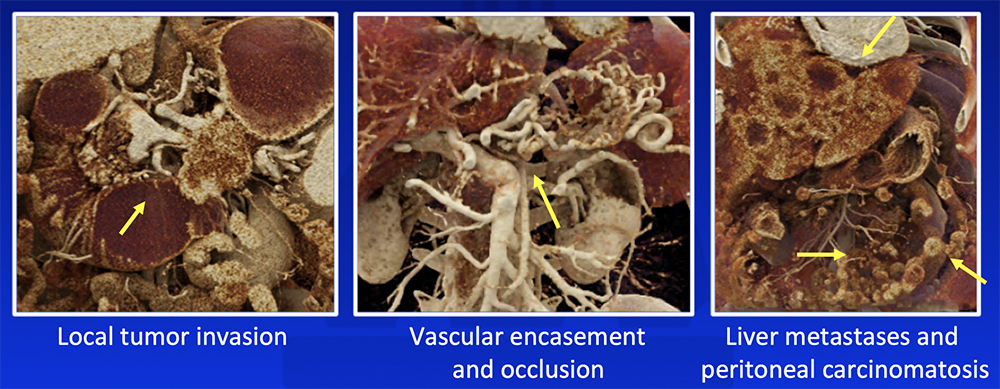 Chu LC et al. Abdom Radiol (NY). 2018:43(11):3009-3015. |
Current State-of-the-Art 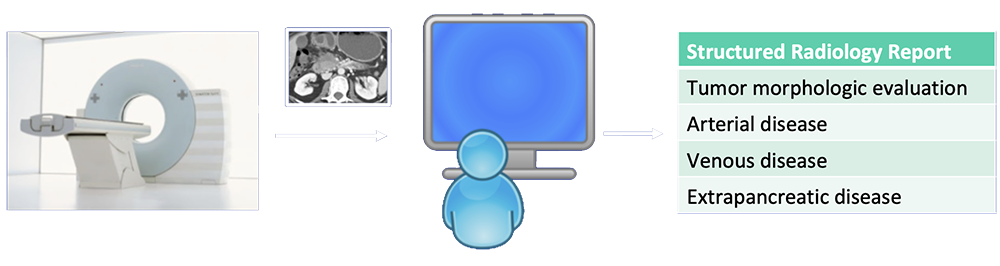 Radiologists rely on visual cues to detection and stage pancreatic tumors. |
Our Vision of Future of Pancreatic Imaging 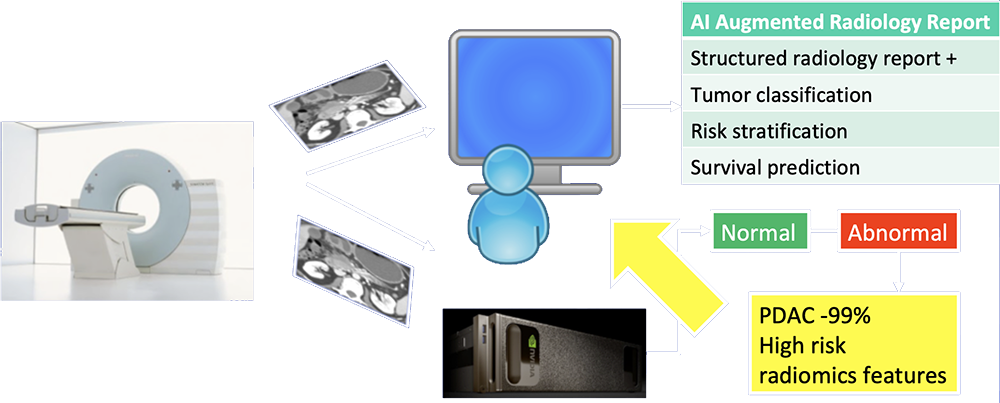 AI functions as second-reader to differentiate abnormal from normal cases, provide probabilities of most likely diagnosis, and extract imaging biomarkers relevant to patient management. |
Conclusions
|
Conclusions
|
References
Acknowledgements
|
