Application of Radiomics in Pancreatic Imaging – Current Status and Future Directions
Application of Radiomics in Pancreatic Imaging - Current Status and Future Directions Linda C. Chu The Russel H. Morgan Department of Radiology and Radiological Science, The Department of Pathology, The Department of Cancer Research, and the Department of Computer Science, Johns Hopkins University, Baltimore |
Disclosure
|
Learning Objectives
|
Introduction
Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics
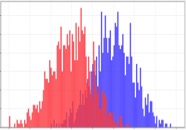 First-Order Statistics Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Aerts HJ et al. Nat Commun. 2014;5:4006. Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics
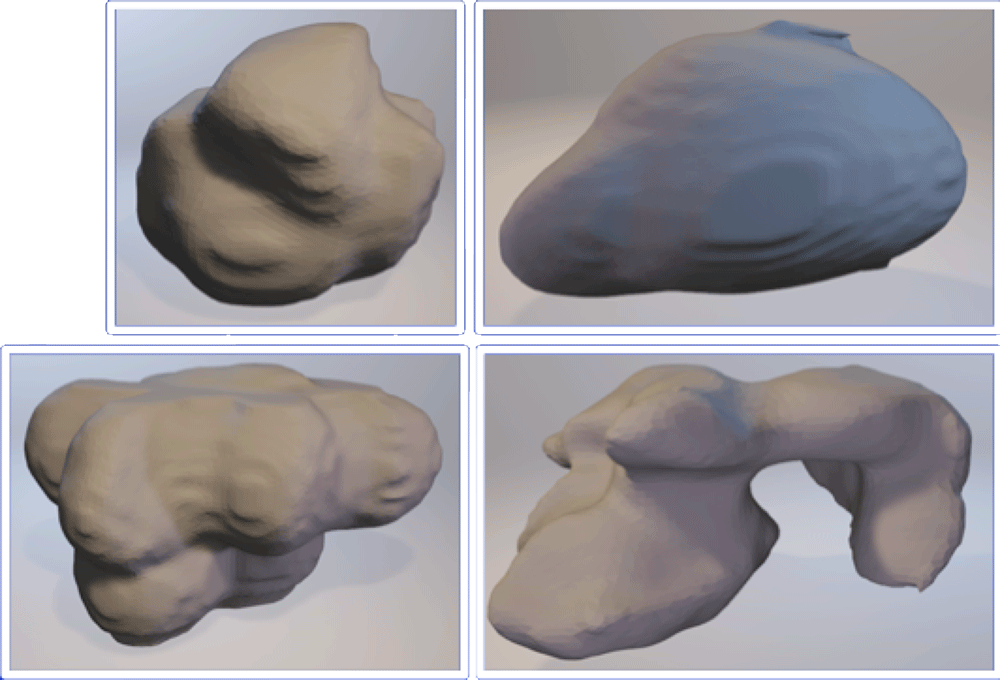 Extracted Shape Representations Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Aerts HJ et al. Nat Commun. 2014;5:4006. Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics
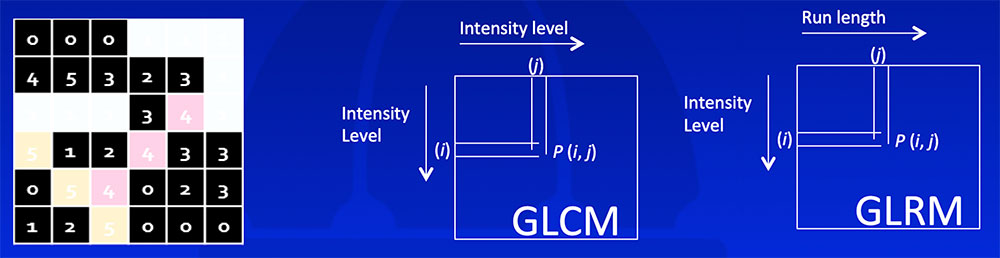 Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Aerts HJ et al. Nat Commun. 2014;5:4006. Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics
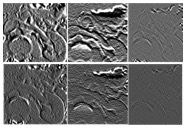  Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Aerts HJ et al. Nat Commun. 2014;5:4006. Gillies RJ et al. Radiology. 2016;278(2):563-577. |
Radiomics – Pancreatic Applications
|
Detection of PDAC
|
Detection of PDAC
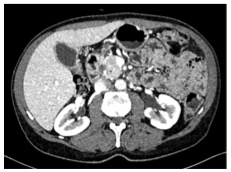 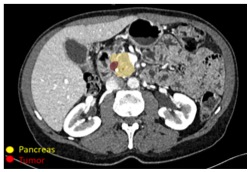 |
Detection of PDAC
|
Detection of PDAC
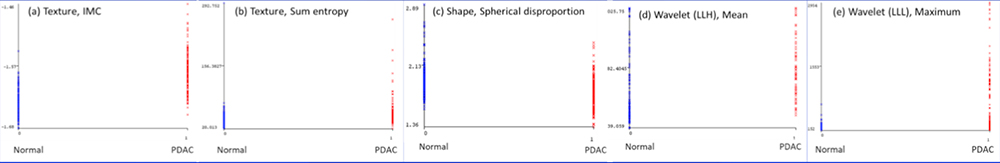 |
Classification
|
Differentiation of Autoimmune Pancreatitis from PDAC
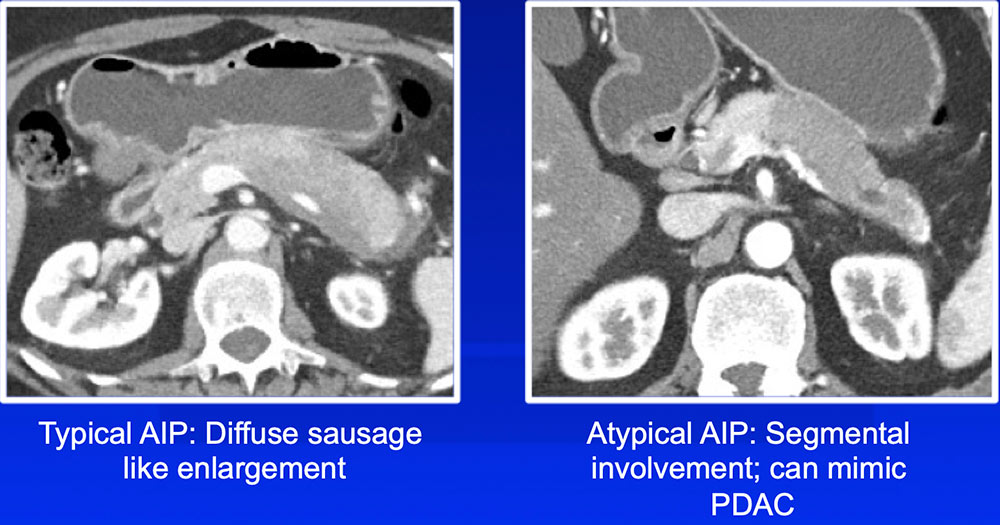 |
Differentiation of Autoimmune Pancreatitis from PDAC
|
Differentiation of Autoimmune Pancreatitis from PDAC
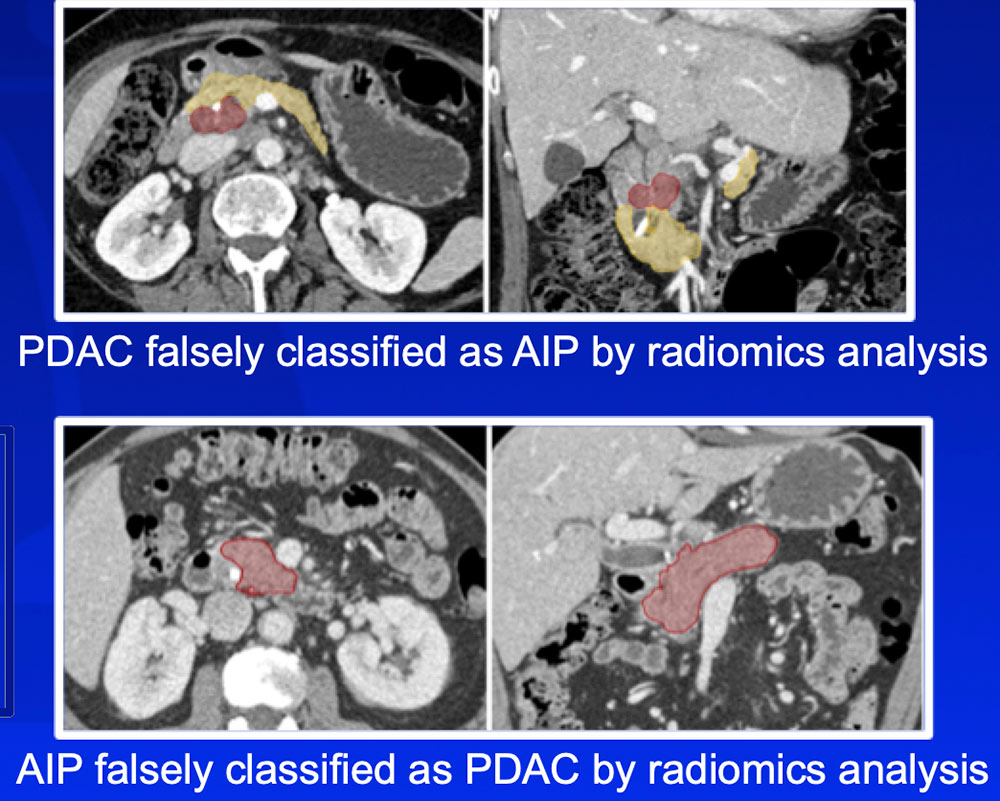 Radiomics features can differentiate AIP from PDAC, which has important treatment implications |
Differentiation of PNET vs. PDAC vs. IPAS
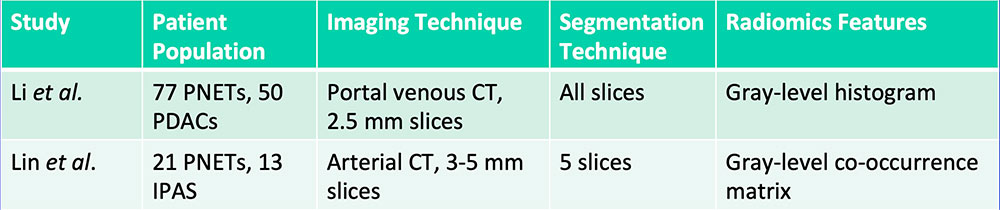 Li J et al. Cancer Med. 2018. [Epub ahead of print]. Lin X et al. Acta Radiol. 2018. [Epub ahead of print] |
Differentiation of PNET vs. PDAC vs. IPAS 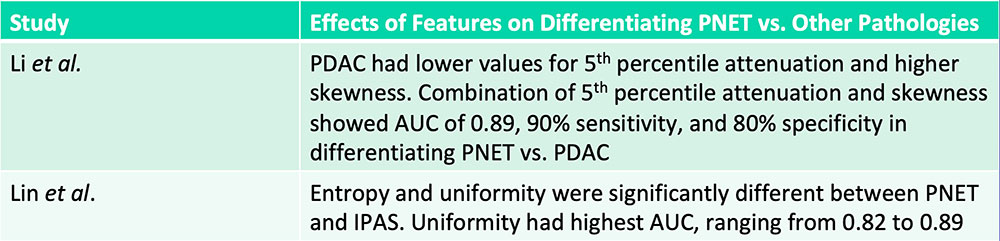 Radiomics features can aid in differentiation of pancreatic neuroendocrine tumors from mimickers such as pancreatic ductal adenocarcinoma and intrapancreatic accessory spleen Li J et al. Cancer Med. 2018. [Epub ahead of print]. Lin X et al. Acta Radiol. 2018. [Epub ahead of print] |
Classification of Pancreatic Cysts
|
Classification of Pancreatic Cysts
|
Classification of Pancreatic Cysts
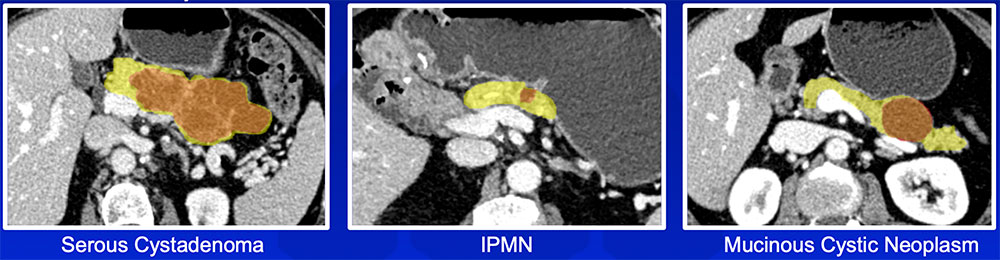 Radiomics features can achieve improved accuracy in classification of pancreatic cystic neoplasms compared to an experienced radiologist |
Differentiation of IPMNs with High-Grade vs. Low-Grade Dysplasia 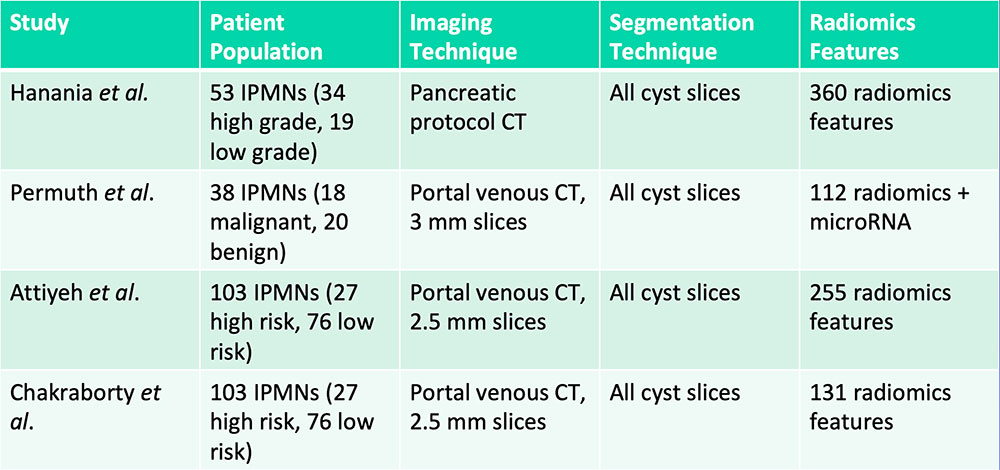 Hanania AN et al. Oncotarget. 2016;7(52):85776-85784. Permuth JB et al. Oncotarget. 2016;7(52):85785-85797. Attiyeh MA et al. HPB (Oxford). 2018 [Epub ahead of print]. Chakraborty J et al. Med Phys. 2018 [Epub ahead of print] |
Differentiation of IPMNs with High-Grade vs. Low-Grade Dysplasia 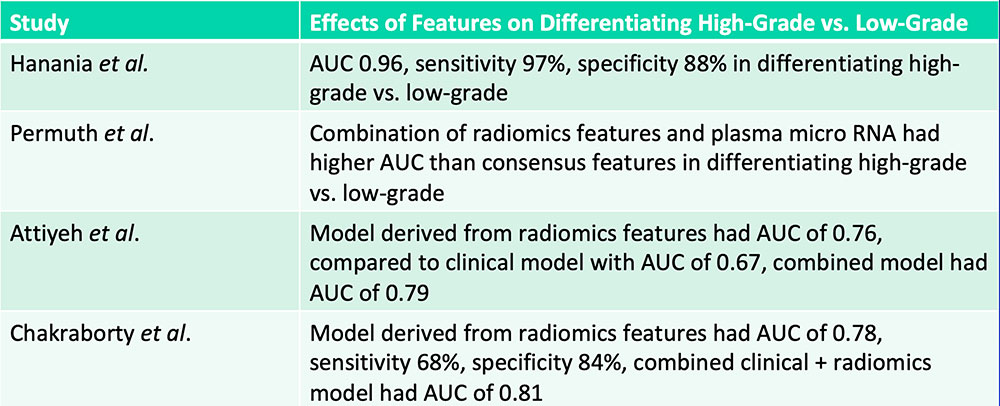 Radiomics features can aid in differentiation of IPMNs with high-grade vs. low-grade dysplasia Hanania AN et al. Oncotarget. 2016;7(52):85776-85784. Permuth JB et al. Oncotarget. 2016;7(52):85785-85797. Attiyeh MA et al. HPB (Oxford). 2018 [Epub ahead of print]. Chakraborty J et al. Med Phys. 2018 [Epub ahead of print] |
Differentiation of Pancreatic Neuroendocrine Tumor Grades 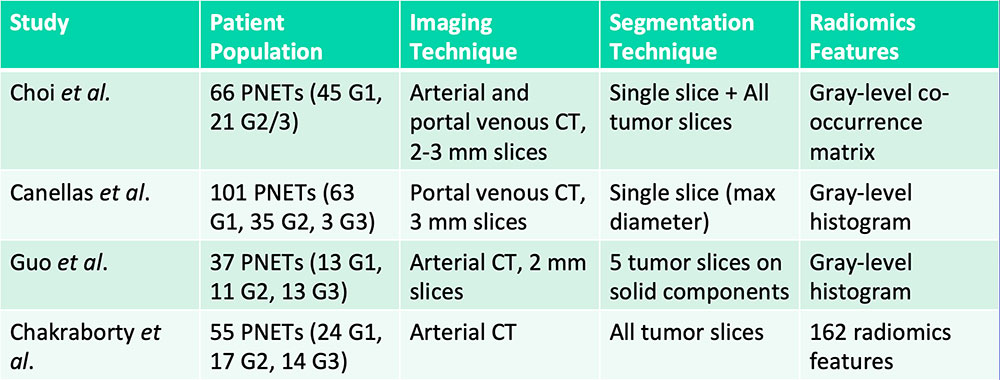 G= Grade Choi TW et al. Acta Radiol. 2018;59(4):383-392. Canellas R et al. AJR. 2018;210(2):341-346. Guo C et al. Abdom Radiol (NY). 2018 [Epub ahead of print]. Chakraborty J et al. SPIE Medical Imaging, Houston, TX. 2018. |
Differentiation of Pancreatic Neuroendocrine Tumor Grades 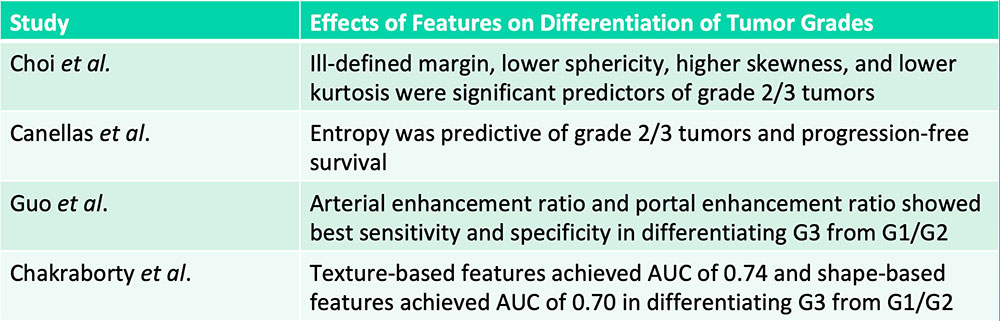 G= Grade Radiomics features can aid in the differentiation of high-grade vs. low-grade pancreatic neuroendocrine tumors Choi TW et al. Acta Radiol. 2018;59(4):383-392. Canellas R et al. AJR. 2018;210(2):341-346. Guo C et al. Abdom Radiol (NY). 2018 [Epub ahead of print]. Chakraborty J et al. SPIE Medical Imaging, Houston, TX. 2018. |
Prognostication – Patient Survival
|
Prognostication – Patient Survival 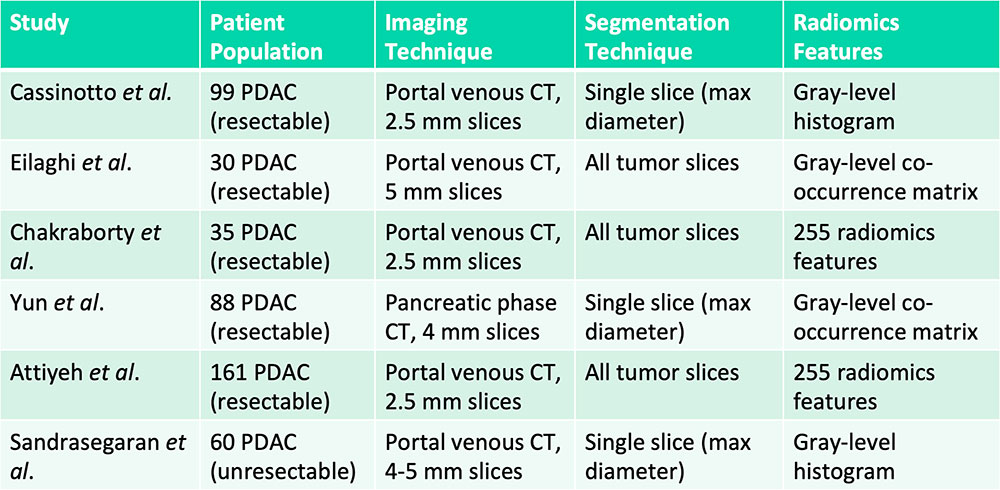 Cassinotto C et al. Eur J Radiol. 2017;90:152-158. Eilaghi A et al. BMC Medical Imaging. 2017;17(1):38. Chakraborty J et al. PLoS One. 2017;12(12):e0188022. Yun G et al. Scientific Reports. 2018;8:7226. Attiyeh MA et al. Ann Surg Oncol. 2018;25:1034-1042. Sandrasegaran K et al. Eur Radiol. 2018 [Epub ahead of print] |
Tumor hypoattenuation and tumor heterogeneity are associated with poor survival in patients with PDAC 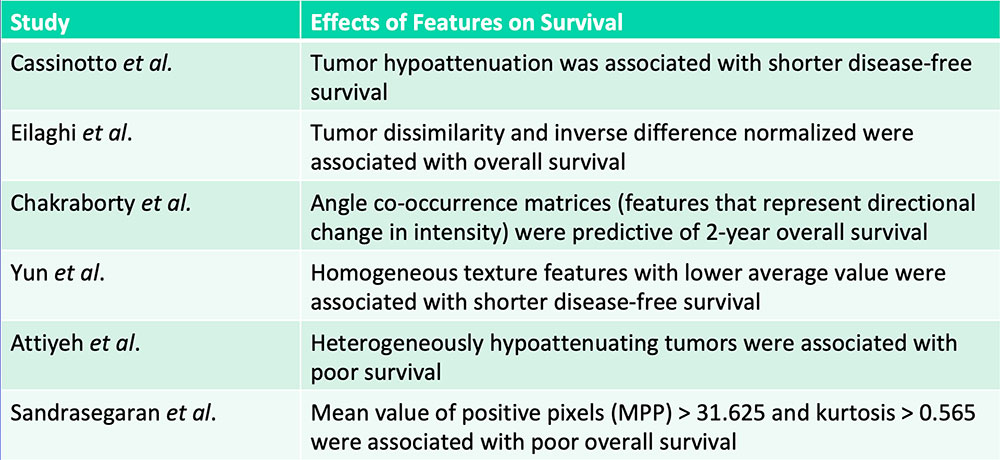 Cassinotto C et al. Eur J Radiol. 2017;90:152-158. Eilaghi A et al. BMC Medical Imaging. 2017;17(1):38. Chakraborty J et al. PLoS One. 2017;12(12):e0188022. Yun G et al. Scientific Reports. 2018;8:7226. Attiyeh MA et al. Ann Surg Oncol. 2018;25:1034-1042. Sandrasegaran K et al. Eur Radiol. 2018 [Epub ahead of print] |
Prognostication – Treatment Response
|
Prognostication – Treatment Response
|
Current Challenges in Radiomics
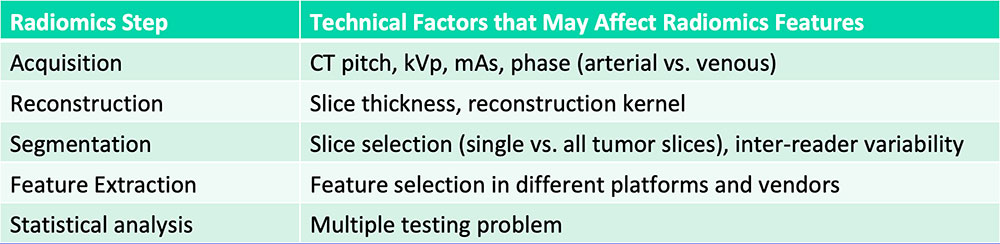 Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. |
Future Directions of Radiomics
|
Conclusion
|
References Aerts HJ et al. Nat Commun. 2014;5:4006. Attiyeh MA et al. Ann Surg Oncol. 2018;25:1034-1042. Attiyeh MA et al. HPB (Oxford). 2018 [Epub ahead of print] Canellas R et al. AJR. 2018;210(2):341-346. Cassinotto C et al. Eur J Radiol. 2017;90:152-158. Chakraborty J et al. PLoS One. 2017;12(12):e0188022. Chakraborty J et al. Med Phys. 2018 [Epub ahead of print] Chakraborty J et al. SPIE Medical Imaging, Houston, TX. 2018. Chan X et al. PLoS One. 2017;12(6):e0178961. Choi TW et al. Acta Radiol. 2018;59(4):383-392. Eilaghi A et al. BMC Medical Imaging. 2017;17(1):38. Gillies RJ et al. Radiology. 2016;278(2):563-577. Guo C et al. Abdom Radiol (NY). 2018 [Epub ahead of print] Hanania AN et al. Oncotarget. 2016;7(52):85776-85784. Kumar V et al. Magn Reson Imaging. 2012;30(9):4-48. Laffan TA et al. AJR. 2008;191:802-807. Li J et al. Cancer Med. 2018. [Epub ahead of print] Lin X et al. Acta Radiol. 2018. [Epub ahead of print] Lubner MG et al. Radiographics. 2017;37(5):1483-1503. Permuth JB et al. Oncotarget. 2016;7(52):85785-85797. Sandrasegaran K et al. Eur Radiol. 2018 [Epub ahead of print] Yun G et al. Scientific Reports. 2018;8:7226. Zanini N et al. Pancreatology. 2015;15:417-422. Acknowledgements
|
